Volume 13, Number 17: 28 April 2010
In a special issue of Oceanography published in December of 2009, Feely et al. review what we supposedly know about the current pH status of the world's oceans, as well as what they say we can likely expect by the end of the current century.
Getting right to the crux of the matter, the three researchers write in their abstract that "estimates based on the Intergovernmental Panel on Climate Change business-as-usual emission scenarios suggest that atmospheric CO2 levels could approach 800 ppm near the end of the century," and that "corresponding biogeochemical models for the ocean indicate that surface water pH will drop from a pre-industrial value of about 8.2 to about 7.8 in the IPCC A2 scenario by the end of this century." And, of course, they warn that, as a result, "the skeletal growth rates of calcium-secreting organisms will be reduced," ending with the obligatory statement that "if anthropogenic CO2 emissions are not dramatically reduced in the coming decades, there is the potential for direct and profound impacts on our living marine ecosystems."
Well that's Feely et al.'s story; but in the very same issue of Oceanography -- in the article that appears just before their paper, in fact -- NOAA's Pieter Tans presents a much different take on the subject.
Tans begins his analysis by indicating that the effect of CO2 on climate -- and, as we shall see -- on its own concentration in the atmosphere -- "depends primarily on the total amount emitted, not on the rate of emissions," and that "unfortunately, the IPCC reports have not helped public understanding of this fact by choosing, somewhat arbitrarily, a rather short time horizon (100 years is most commonly used) for climate forcing by CO2." Thus, "instead of adopting the common economic point of view, which, through its emphasis on perpetual growth, implicitly assumes infinite earth resources," Tans notes that the cumulative extraction of fossil-fuel carbon currently stands at about 345 GtC, and that there appears to be another 640 or so GtC of proven reserves, yielding a total original reserve of about 1,000 GtC, from which he proceeds with his analysis.
The figure below shows much of the past and projected history of fossil-fuel carbon utilization, together with historical and projected atmospheric CO2 concentrations out to the year 2500, as calculated by Tans. As can be seen there, his analysis indicates that the air's CO2 concentration peaks well before 2100 and at only 500 ppm, as compared to the 800 ppm that Feely et al. take from the IPCC. In addition, by the time the year 2500 rolls around, the air's CO2 concentration actually drops back to about what it is today.
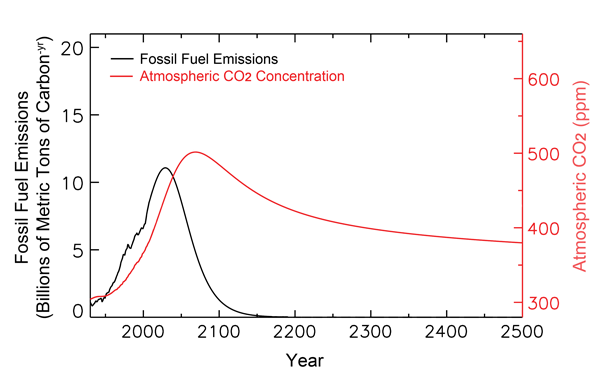
Figure 1. Past and projected trends of fossil-fuel carbon utilization and the atmosphere's CO2 concentration. Adapted from Tans (2009).
Based on his more modest projections of future atmospheric CO2 concentrations, Tans also finds the projected pH reduction of ocean waters in the year 2100 (as compared to preindustrial times) to be only one-half of the 0.4 value calculated by Feely et al., with a recovery to a reduction of only a tad over 0.1 pH unit by 2500, which is less than the range of pH values that are typical of today's oceans (8.231 in the Arctic Ocean minus 8.068 in the North Indian Ocean equals 0.163, according to Feely et al.).
Clearly, things are not the way the world's climate alarmists make them out to be, especially when it comes to potential effects of anthropogenic CO2 emissions and their effects on the air's CO2 content and oceanic pH values.
Sherwood, Keith and Craig Idso
References
Feely, R.A., Doney, S.C. and Cooley, S.R. 2009. Ocean acidification: Present conditions and future changes in a high-CO2 world. Oceanography 22: 36-47.
Tans, P. 2009. An accounting of the observed increase in oceanic and atmospheric CO2 and an outlook for the future. Oceanography 22: 26-35.




