CO2, Global Warming and Coral Reefs
Part II: Direct Threats
In addition to the CO2-induced indirect threats postulated to harm the world's coral reefs, as discussed in Part 1 of this document, the global increase in the atmosphere's CO2 content has been hypothesized to possess the potential to harm coral reefs directly. By inducing changes in ocean water chemistry that can lead to reductions in the calcium carbonate saturation state of seawater, it has been predicted that elevated levels of atmospheric CO2 may reduce rates of coral calcification, possibly leading to slower-growing – and, therefore, weaker – coral skeletons, and in some cases even death.
We begin this part of our review by discussing the important role biology plays in driving the physical-chemical process of coral calcification, followed by a discussion of several real-world observations that depict increasing rates of coral calcification in the face of rising temperatures and atmospheric CO2 concentrations. Indeed, as ever more pertinent evidence accumulates, the true story appears to be just the opposite of what the ocean acidification hypothesis promotes.
1. Ocean Acidification
The rate of deposition of calcium carbonate on coral reefs, or coral calcification rate, is controlled at the cellular level by the saturation state of calcium carbonate in seawater; and oceanic surface waters have likely been saturated or supersaturated in this regard – providing a good environment for coral reef growth – since early Precambrian times (Holland, 1984). Currently, however, as the air’s CO2 content rises in response to ever-increasing anthropogenic CO2 emissions, and as more and more carbon dioxide therefore dissolves in the surface waters of the world’s oceans, pH values of the planet’s oceanic waters are, or should be, gradually dropping, leading to a reduction in the calcium carbonate saturation state of seawater.
This phenomenon has been theorized to be leading to a corresponding reduction in coral calcification rates (Smith and Buddemeier, 1992; Buddemeier, 1994; Buddemeier and Fautin, 1996a,b; Holligan and Robertson, 1996; Gattuso et al., 1998; Buddemeier and Smith, 1999; IPCC, 2007a,b; De'ath et al., 2009), which reduction has been hypothesized to be rendering corals more susceptible to a number of other environmental stresses, including “sea-level rise, extreme temperatures, human damage (from mining, dredging, fishing and tourism), and changes in salinity and pollutant concentrations (nutrients, pesticides, herbicides and particulates), and in ocean currents, ENSO, and storm damage” (Pittock, 1999). Kleypas et al. (1999), for example, have calculated that calcification rates of tropical corals should already have declined by 6 to 11% or more since 1880, as a result of the concomitant increase in atmospheric CO2 concentration; and they predict that the reductions could reach 17 to 35% by 2100, as a result of expected increases in the air's CO2 content over the coming century. Likewise, Langdon et al. (2000) calculated a decrease in coral calcification rate of up to 40% between 1880 and 2065.
The ocean chemistry aspect of this theory is rather straightforward; but it certainly is not as solid as acidification alarmists make it out to be. In evaluating global seawater impacts of (1) model-predicted global warming and (2) direct seawater chemical consequences of a doubling of the air's CO2 content, Loaiciga (2006), for example, used a mass-balance approach to (1) "estimate the change in average seawater salinity caused by the melting of terrestrial ice and permanent snow in a warming earth," and he (2) applied "a chemical equilibrium model for the concentration of carbonate species in seawater open to the atmosphere" in order to "estimate the effect of changes in atmospheric CO2 on the acidity of seawater." Assuming that the rise in the planet's mean surface air temperature continues unabated, and that it eventually causes the melting of all terrestrial ice and permanent snow, Loaiciga calculated that "the average seawater salinity would be lowered not more than 0.61‰ from its current 35‰." He also reports that across the range of seawater temperature considered (0 to 30°C), "a doubling of CO2 from 380 ppm to 760 ppm increases the seawater acidity [lowers its pH] approximately 0.19 pH units." He thus concludes that "on a global scale and over the time scales considered (hundreds of years), there would not be accentuated changes in either seawater salinity or acidity from the rising concentration of atmospheric CO2."
Furthermore, with more CO2 in the air, additional weathering of terrestrial carbonates is likely to occur, which would increase delivery of Ca2+ to the oceans and partly compensate for the CO2-induced decrease in oceanic calcium carbonate saturation state (Riding, 1996). And as with all phenomena involving living organisms, the introduction of life into the ocean acidification picture greatly complicates things. Considerations of a suite of interrelated biological phenomena, for example, also make it much more difficult to draw such sweeping negative conclusions as are currently being discussed. Indeed, as shown in the next section, they even suggest that the rising CO2 content of earth’s atmosphere may well be a positive phenomenon, enhancing thegrowth rates of coral reefs and helping them to better withstand the many environmental stresses that truly are inimical to their well-being.
1.1. The Important Role of Biology
Over half a century ago, Kawaguti and Sakumoto (1948) illustrated the important role played by photosynthesis in the construction of coral reefs. Specifically, they analyzed numerous data sets recorded in several earlier publications, demonstrating that coral calcification rates are considerably higher in the daylight (when photosynthesis by coral symbionts occurs) than they are in the dark (when the symbionts lose carbon via respiration). A number of more modern studies have also demonstrated that symbiont photosynthesis enhances coral calcification (Barnes and Chalker, 1990; Yamashiro, 1995); and they have further demonstrated that long-term reef calcification rates generally rise in direct proportion to increases in rates of reef primary production (Frankignoulle et al., 1996; Gattuso et al., 1996, 1999). In fact, the work of Muscatine (1990) suggests that “the photosynthetic activity of zooxanthellae is the chief source of energy for the energetically expensive process of calcification” (Hoegh-Guldberg, 1999). Consequently, if an anthropogenic-induced increase in the transfer of CO2 from the atmosphere to the world’s oceans, i.e., hydrospheric CO2 enrichment, were to lead to increases in coral symbiont photosynthesis – as atmospheric CO2 enrichment does for essentially all terrestrial plants (Kimball, 1983; Idso, 1992) – it is likely that increases in coral calcification rates would occur as well.
There are several reasons for expecting a positive coral calcification response to CO2-enhanced symbiont photosynthesis. One of the first mechanisms to come to mind is the opposite of the phenomenon that has been proffered as a cause of future declines in coral calcification rates. This reverse phenomenon is the decrease in extracellular CO2 partial pressure in coral tissues that is driven by the drawdown of aqueous CO2 caused by the photosynthetic process. With CO2 being removed from the water in intimate contact with the coral host via its fixation by photosynthesis (which CO2 drawdown is of far greater significance to the coral than the increase in the CO2 content of the surrounding bulk water that is affected by the ongoing rise in the air’s CO2 content), the pH and calcium carbonate saturation state of the water immediately surrounding the coral host should rise (Goreau, 1959), enhancing the coral’s calcification rate (Gattuso et al., 1999). And if hydrospheric CO2 enrichment stimulates zooxanthellae photosynthesis to the same degree that atmospheric CO2 enrichment stimulates photosynthesis in terrestrial plants, i.e., by 30 to 50% for a 300 ppm increase in CO2 concentration (Kimball, 1983; Idso 1992, Idso and Idso, 1994), this phenomenon alone would more than compensate for the drop in the calcium carbonate saturation state of the bulk-water of the world’s oceans produced by the ongoing rise in the air’s CO2 content, which Gattuso et al. (1999) have calculated could lead to a 15% reduction in coral calcification rate for a doubling of the pre-industrial atmospheric CO2 concentration.
Another reason why coral calcification may proceed at a higher rate in the presence of CO2-stimulated symbiont photosynthesis is that, while growing more robustly, the zooxanthellae may take up more of the metabolic waste products of the coral host, which, if present in too great quantities, can prove detrimental to the health of the host, as well as the health of the entire coral plant-animal assemblage (Yonge, 1968; Crossland and Barnes, 1974). There are also a number of other substances that are known to directly interfere with calcium carbonate precipitation; and they too can be actively removed from the water by coral symbionts in much the same way that symbionts remove host waste products (Simkiss, 1964). More importantly, perhaps, a greater amount of symbiont-produced photosynthates may provide more fuel for the active transport processes involved in coral calcification (Chalker and Taylor, 1975), as well as more raw materials for the synthesis of the coral organic matrix (Wainwright, 1963; Muscatine, 1967; Battey and Patton, 1984). Finally, the photosynthetic process helps to maintain a healthy aerobic or oxic environment for the optimal growth of the coral animals (Rinkevich and Loya, 1984; Rands et al., 1992); and greater CO2-induced rates of symbiont photosynthesis would enhance this important “environmental protection activity.”
Such observations invoke a number of questions. With ever more CO2 going into the air, driving ever more CO2 into the oceans, might we not logically expect to see increasingly greater rates of coral symbiont photosynthesis, due to the photosynthesis-stimulating effect of hydrospheric CO2 enrichment? Would not this phenomenon, in turn, increasingly enhance all of the many positive photosynthetic-dependent phenomena we have described and thereby increase coral calcification rates? And might it not increase these rates well beyond the point of overpowering the modest negative effect of the purely chemical consequences of elevated dissolved CO2 on ocean pH and calcium carbonate saturation state?
The answers to these several questions are probably all “yes,” but arriving at these conclusions is not as simple as it sounds. For one thing, although many types of marine plant life do indeed respond to hydrospheric CO2 enrichment (Raven et al., 1985) – including seagrasses (Zimmerman et al., 1997), certain diatoms (Riebesell et al., 1993; Chen and Gao, 2004; Sobrino et al., 2008), macroalgae (Borowitzka and Larkum, 1976; Gao et al., 1993a), and microalgae or phytoplankton (Raven, 1991; Nimer and Merrett, 1993) – the photosynthesis of many marine autotrophs is normally not considered to be carbon-limited, because of the large supply of bicarbonate in the world’s oceans (Raven, 1997). However, as Gattuso et al. (1999) explain, this situation is only true for autotrophs that possess an effective carbon-concentrating mechanism; but to swing once again in the other direction, it is also believed that many coral symbionts are of this type (Burris et al., 1983; Al-Moghrabi et al., 1996; Goiran et al., 1996).
Nevertheless, in yet another positive twist to this complex story, Gattuso et al. (1999) report that coral zooxanthellae – in another grand example of adaptation – are able to change their mechanism of carbon supply in response to various environmental stimuli. Furthermore, Beardall et al. (1998) suggest that an increased concentration of dissolved CO2, together with an increase in the rate of CO2 generation by bicarbonate dehydration in host cells, may favor a transition to the diffusional mode of carbon supply, which is sensitive to hydrospheric CO2 concentration. Consequently, if such a change in mode of carbon supply were to occur – prompted, perhaps, by hydrospheric CO2 enrichment itself – this shift in CO2 fixation strategy would indeed allow the several biological mechanisms we have described to operate to enhance reef calcification rates in response to a rise in the air’s CO2 content.
In one final example that demonstrates the importance of biology in driving the physical-chemical process of coral calcification, Muscatine et al. (2005) note that the "photosynthetic activity of zooxanthellae is the chief source of energy for the energetically-expensive process of calcification," and that long-term reef calcification rates have generally been observed to rise in direct proportion to increases in rates of reef primary production, which they say may well be enhanced by increases in the air's CO2 concentration.
Muscatine et al. begin the report of their investigation of the subject by stating much the same thing, i.e., that endosymbiotic algae "release products of photosynthesis to animal cells ... and augment the rate of skeletal calcification." Then, noting that the "natural abundance of stable isotopes (δ13C and δ15N) has answered paleobiological and modern questions about the effect of photosymbiosis on sources of carbon and oxygen in coral skeletal calcium carbonate," they go on to investigate the natural abundance of these isotopes in another coral skeletal compartment - the skeletal organic matrix (OM) - in 17 species of modern scleractinian corals, after which they compare the results for symbiotic and nonsymbiotic forms to determine the role played by algae in OM development.
Why is this study an important scientific undertaking? It is because, in the words of Muscatine et al., the scleractinian coral skeleton is a two-phase composite structure consisting of fiber-like crystals of aragonitic calcium carbonate intimately associated with an intrinsic OM," and although the OM generally comprises less than 0.1% of the total weight of the coral skeleton, it is, in their words, "believed to initiate nucleation of calcium carbonate and provide a framework for crystallographic orientation and species-specific architecture." In fact, they say that inhibition of OM synthesis "brings coral calcification to a halt."
So what did Muscatine et al. learn from their experiments? They say their "most striking observation is the significant difference in mean OM δ15N between symbiotic and nonsymbiotic corals," which makes OM δ15N an important proxy for photosymbiosis. As an example of its usefulness, they applied the technique to a fossil coral (Pachythecalis major) from the Triassic (which prevailed some 240 million years ago), finding that the ancient coral was indeed photosymbiotic. Even more importantly, however, they conclude in the final sentence of their paper that "it now seems that symbiotic algae may control calcification by both modification of physico-chemical parameters within the coral polyps (Gautret et al., 1997; Cuif et al., 1999) and augmenting the synthesis of OM (Allemand et al., 1998)."
Yes, in some respects it is true that life is at the mercy of the elements, but in other respects it is equally true that life rules.
Although lacking the research to absolutely identify the “what” and definitively describe the “how” of the hypothesis of hydrospheric CO2 enhancement of coral calcification, it is clear that something of the nature described above can indeed act to overcome the negative effect of the high-CO2-induced decrease in calcium carbonate saturation state on coral calcification rate. It has been clearly demonstrated, for example, that corals can grow quite well in aquariums containing water of very high dissolved CO2 concentration (Atkinson et al., 1995); and Carlson (1999) has stated that the fact that corals often thrive in such water “seems to contradict conclusions ... that high CO2 may inhibit calcification.” And there are numerous other examples of such phenomena in the real world of nature, which we examine next.
1.2. Coral Calcification
Many are the people who have predicted that rates of coral calcification, as well as the photosynthetic rates of their symbiotic algae, will dramatically decline in response to what they typically refer to as an acidification of the world's oceans, as the atmosphere's CO2 concentration continues to rise in the years, decades and centuries to come. As ever more pertinent evidence accumulates, however, the true story appears to be just the opposite of what these acidification alarmists continue to tell us.
We begin with the recent study of Herfort et al. (2008), who note that an increase in atmospheric CO2 will cause an increase in the abundance of HCO3- (bicarbonate) ions and dissolved CO2, and who report that several studies on marine plants have observed "increased photosynthesis with higher than ambient DIC [dissolved inorganic carbon] concentrations," citing the works of Gao et al. (1993b), Weis (1993), Beer and Rehnberg (1997), Marubini and Thake (1998), Mercado et al. (2001, 2003), Herfort et al. (2002) and Zou et al. (2003).
To further explore this subject, and to see what it might imply for coral calcification, the three researchers employed a wide range of bicarbonate concentrations "to monitor the kinetics of bicarbonate use in both photosynthesis and calcification in two reef-building corals, Porites porites and Acropora sp." This work revealed that additions of HCO3- to synthetic seawater continued to increase the calcification rate of Porites porites until the bicarbonate concentration exceeded three times that of seawater, while photosynthetic rates of the coral's symbiotic algae were stimulated by HCO3- addition until they became saturated at twice the normal HCO3- concentration of seawater.
Similar experiments conducted on Indo-Pacific Acropora sp. showed that calcification and photosynthetic rates in these corals were enhanced to an even greater extent, with calcification continuing to increase above a quadrupling of the HCO3- concentration and photosynthesis saturating at triple the concentration of seawater. In addition, they monitored calcification rates of the Acropora sp. in the dark, and, in their words, "although these were lower than in the light for a given HCO3- concentration, they still increased dramatically with HCO3- addition, showing that calcification in this coral is light stimulated but not light dependent."
In discussing the significance of their findings, Herfort et al. suggest that "hermatypic corals incubated in the light achieve high rates of calcification by the synergistic action of photosynthesis [our italics]," which, as they have shown, is enhanced by elevated concentrations of HCO3- ions that come courtesy of the ongoing rise in the air's CO2 content. As for the real-world implications of their work, the three researchers note that over the next century the predicted increase in atmospheric CO2 concentration "will result in about a 15% increase in oceanic HCO3-," and they say that this development "could stimulate photosynthesis and calcification in a wide variety of hermatypic corals," a conclusion that stands in stark contrast to the contention of the world's acidification alarmists.
In another study, Pelejero et al. (2005) developed a reconstruction of seawater pH spanning the period 1708-1988, based on the boron isotopic composition (ä11B) of a long-lived massive coral (Porites) from Flinders Reef in the western Coral Sea of the southwestern Pacific. Results indicated that "there [was] no notable trend toward lower δ11B values" over the 300-year period investigated. Instead, they say that "the dominant feature of the coral δ11B record is a clear interdecadal oscillation of pH, with δ11B values ranging between 23 and 25 per mil (7.9 and 8.2 pH units)," which "is synchronous with the Interdecadal Pacific Oscillation." Furthermore, they calculated changes in aragonite saturation state from the Flinders pH record that varied between ~3 and 4.5, which values encompass "the lower and upper limits of aragonite saturation state within which corals can survive." Despite this fact, they report that "skeletal extension and calcification rates for the Flinders Reef coral fall within the normal range for Porites and are not correlated with aragonite saturation state or pH [our italics]."
Thus, contrary to acidification-alarmist claims that historical anthropogenic CO2 emissions have already resulted in a significant decline in ocean water pH and aragonite saturation state, Pelejero et al.'s 300-year record of these parameters (which, in their words, began "well before the start of the Industrial Revolution") provides no evidence of such a decline. What is more, and also contrary to what one would expect from climate-alarmist claims of how sensitive coral calcification rate is to changes in pH and aragonite saturation state, they found that huge cyclical changes in these parameters had essentially no detectable effect on either coral calcification or skeletal extension rates.
Moving a little backward in time, in a study of historical calcification rates determined from coral cores retrieved from 35 sites on the Great Barrier Reef, Lough and Barnes (1997) observed a statistically significant correlation between coral calcification rate and local water temperature, such that a 1°C increase in mean annual water temperature increased mean annual coral calcification rate by about 3.5%. Nevertheless, they report there were "declines in calcification in Porites on the Great Barrier Reef over recent decades." They are quick to point out, however, that their data depict several extended periods of time when coral growth rates were either above or below the long-term mean, cautioning that "it would be unwise to rely on short-term values (say averages over less than 30 years) to assess mean conditions."
As an example of this fact, they report that "a decline in calcification equivalent to the recent decline occurred earlier this century and much greater declines occurred in the 18th and 19th centuries," long before anthropogenic CO2 emissions made much of an impact on the air's CO2 concentration. In fact, over the entire expanse of their data set, Lough and Barnes say "the 20th century has witnessed the second highest period of above average calcification in the past 237 years," which is not exactly what one would expect in light of (1) how dangerous high water temperatures are often said to be for corals, (2) the climate-alarmist claim that earth is currently warmer than it has been at any other time during the entire past millennium, and (3) the fact that the air's CO2 content is currently much higher than it has been for far longer than a mere thousand years.
Similar findings were reported by Bessat and Buigues (2001), who derived a history of coral calcification rates from a core extracted from a massive Porites coral head on the French Polynesian island of Moorea that covered the period 1801-1990. They performed this work, they say, because "recent coral-growth models highlight the enhanced greenhouse effect on the decrease of calcification rate," and rather than relying on theoretical calculations, they wanted to work with real-world data, stating that the records preserved in ancient corals "may provide information about long-term variability in the performance of coral reefs, allowing unnatural changes to be distinguished from natural variability."
So what did Bessat and Buigues learn? First of all, they found that a 1°C increase in water temperature increased coral calcification rate at the site they studied by 4.5%. Then, they found that "instead of a 6-14% decline in calcification over the past 100 years computed by the Kleypas group, the calcification has increased, in accordance with [the results of] Australian scientists Lough and Barnes." They also observed patterns of "jumps or stages" in the record, which were characterized by an increase in the annual rate of calcification, particularly at the beginning of the past century "and in a more marked way around 1940, 1960 and 1976," stating once again that their results "do not confirm those predicted by the Kleypas et al. (1999) model."
Another major blow to the Kleypas et al. model was provided by the work of Lough and Barnes (2000), who assembled and analyzed the calcification characteristics of 245 similar-sized massive colonies of Porites corals obtained from 29 reef sites located along the length, and across the breadth, of Australia's Great Barrier Reef (GBR), which data spanned a latitudinal range of approximately 9° and an annual average sea surface temperature (SST) range of 25-27°C. To these data they added other published data from the Hawaiian Archipelago (Grigg, 1981, 1997) and Phuket, Thailand (Scoffin et al., 1992), thereby extending the latitudinal range of the expanded data set to 20° and the annual average SST range to 23-29°C.
This analysis revealed that the GBR calcification data were linearly related to the average annual SST data, such that "a 1°C rise in average annual SST increased average annual calcification by 0.39 g cm-2 year-1." Results were much the same for the extended data set; Lough and Barnes report that "the regression equation [calcification = 0.33(SST) - 7.07] explained 83.6% of the variance in average annual calcification (F = 213.59, p less than 0.00)," noting that "this equation provides for a change in calcification rate of 0.33 g cm-2 year-1 for each 1°C change in average annual SST."
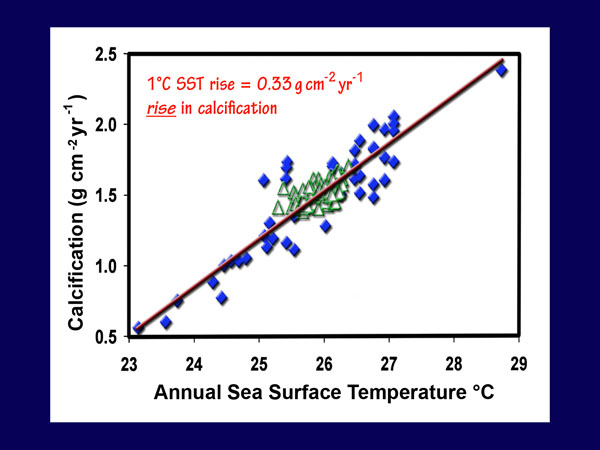
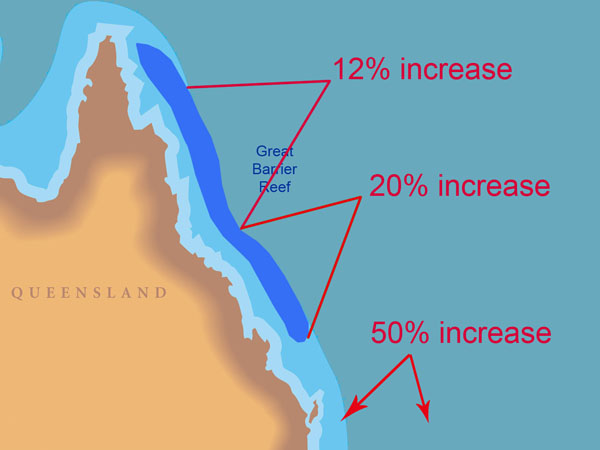 With respect to the significance of their findings, Lough and Barnes say they "allow assessment of possible impacts of global climate change on coral reef ecosystems," and between the two 50-year periods 1880-1929 and 1930-1979, they calculate a calcification increase of 0.06 g cm-2 year-1, noting that "this increase of ~4% in calcification rate conflicts with the estimated decrease in coral calcification rate of 6-14% over the same time period suggested by Kleypas et al. (1999) as a response to changes in ocean chemistry." Even more stunning is their observation that between the two 20-year periods 1903-1922 and 1979-1998, "the SST-associated increase in calcification is estimated to be less than 5% in the northern GBR, ~12% in the central GBR, ~20% in the southern GBR and to increase dramatically (up to ~50%) to the south of the GBR."
With respect to the significance of their findings, Lough and Barnes say they "allow assessment of possible impacts of global climate change on coral reef ecosystems," and between the two 50-year periods 1880-1929 and 1930-1979, they calculate a calcification increase of 0.06 g cm-2 year-1, noting that "this increase of ~4% in calcification rate conflicts with the estimated decrease in coral calcification rate of 6-14% over the same time period suggested by Kleypas et al. (1999) as a response to changes in ocean chemistry." Even more stunning is their observation that between the two 20-year periods 1903-1922 and 1979-1998, "the SST-associated increase in calcification is estimated to be less than 5% in the northern GBR, ~12% in the central GBR, ~20% in the southern GBR and to increase dramatically (up to ~50%) to the south of the GBR."
In light of these real-world observations, and in stark contrast to the doom-and-gloom prognostications of the world's climate alarmists, Lough and Barnes concluded that coral calcification rates "may have already significantly increased along the GBR in response to global climate change." But in spite this compelling evidence, as well as the similar findings of others, claims of impending coral doom caused by rising air temperatures and CO2 concentrations have continued to rear their ugly heads ... on a regular basis ... and in the usual places.
In Nature, it was Caldeira and Wickett (2003) who kept the catastrophe ball rolling. Based on a geochemical model, an ocean general-circulation model, an IPCC CO2 emissions scenario for the 21st century, and a logistic function for the burning of earth's post-21st century fossil-fuel reserves, they calculated three important numbers: the maximum level to which the air's CO2 concentration might rise, the point in time when that might happen, and the related decline that might be expected to occur in ocean-surface pH. These calculations indicated that earth's atmospheric CO2 concentration could approach 2000 ppm around the year 2300, leading to an ocean-surface pH reduction of 0.7 units, a change described by Caldeira and Wickett as being much more rapid and considerably greater "than any experienced in the past 300 million years," which, of course, proves deadly for earth's corals in their scenario.
The following year, similar concerns were aroused by a report prepared for the Pew Center on Global Climate Change, which was released to the public on 13 February 2004 at the annual meeting of the American Association for the Advancement of Science. In that document, Buddemeier et al. (2004) claimed that the projected increase in the air's CO2 content and the simulated decline in ocean-surface pH would dramatically decrease coral calcification rates, which were predicted to lead to "a slow-down or reversal of reef-building and the potential loss of reef structures."
Nevertheless, and because of all the contrary evidence, much of which we have cited above, Buddemeier et al. (2004) were forced to acknowledge that "calcification rates of large heads of the massive coral Porites increased rather than decreased over the latter half of the 20th century," further noting that "temperature and calcification rates are correlated, and these corals have so far responded more to increases in water temperature (growing faster through increased metabolism and the increased photosynthetic rates of their zooxanthellae) than to decreases in carbonate ion concentration."
The most recent claims of impending coral doom derive from the 2009 Science study of De'ath et al., who examined coral calcification rates on the Great Barrier Reef over the past 400 years. Results of their analysis indicate that there was a 14% decline in Porites calcification rate between 1990 and 2005, which observation the authors claim “is unprecedented in at least the past 400 years.” As one might expect, the media’s regurgitations of the these scientists’ findings included some ominous declarations. The headline of a BBC News report, for example, proclaimed “coral reef growth is slowest ever,” while a Sky News headline read “Barrier Reef coral growth ‘will stop’.” And ABC News actually stated when it might stop, concluding their report by quoting the research paper’s senior author as saying “coral growth could hit zero by 2050.”
But how correct are such claims? Beginning with the first claim that “coral reef growth is slowest ever,” ever, in this context, is a very long time, which suggests that for as long as there have been corals to grow the recent decline is unprecedented. So, no, it can’t possibly be right. Or at least it can’t be known to be right. But as we intimated earlier in Part 1 of this document, corals have been around for quite a long time, even longer than all of mankind. In fact, the scleractinian corals, which are the major builders of the reefs of today, have been around some 200 million years, during most of which time both the atmosphere’s CO2 concentration and its temperature were much greater than they are today, which should immediately raise a red flag about the proffered cause of the recent decline in reef growth. And in regard to the recent decline in calcification being unprecedented in the past 400 years, all one needs to do is follow the published De'ath et al. calcification history back in time a mere 33 more years, from 1605 to 1572, to see that claim washed away. For the coral calcification rate during that earlier time was approximately 23% lower than what it was at its 20th-century peak, not to mention the fact that the air’s CO2 concentration was more than 100 ppm less than what it is today and, therefore, supposedly so much more healthier for corals (if you believe climate alarmists!).
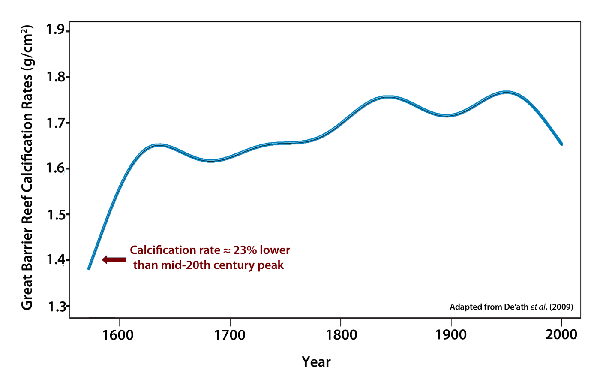
Another way of looking at De’ath et al.’s data is to realize that from 1572 to the 20th century peak, Porites calcification rates on the Great Barrier Reef rose by about 29%, as the “twin evils” of the radical environmentalist movement -- atmospheric CO2 concentration and air temperature -- rose concurrently, after which calcification rates declined, but by a smaller 14%, as these same air temperature and CO2 trends continued, further obfuscating the issue.
But why would anyone believe that the recent calcification decline implies that Porites coral growth “will stop,” and that the end will come “by 2050”? They believe it because certain scientists (such as James Hansen) and politicians (such as Al Gore) imply much the same thing, as even De’ath et al. do. But when they feel compelled to be as correct and as true to their data as possible, such as when writing in Science, the three researchers from the Australian Institute of Marine Science clearly state that “the causes for the Great Barrier Reef-wide decline in coral calcification of massive Porites remain unknown.” And when the causes of the recent decline in coral calcification rate are admitted to be unknown, it seems foolish indeed to predict, not only that the decline will continue, but that it will lead all the way to the demise of the studied coral, and especially at a specified future date, which, we might add, De’ath et al. appropriately do not do in their Science paper.
Moving on, a second good reason for not believing that the ongoing rise in the air's CO2 content will lead to reduced oceanic pH and, therefore, lower calcification rates in the world's coral reefs, is that the same phenomenon that powers the twin processes of coral calcification and phytoplanktonic growth (photosynthesis) tends to increase the pH of marine waters (Gnaiger et al., 1978; Santhanam et al., 1994; Brussaard et al., 1996; Lindholm and Nummelin, 1999; Macedo et al., 2001; Hansen, 2002); and this phenomenon has been shown to have the ability to dramatically increase the pH of marine bays, lagoons and tidal pools (Gnaiger et al., 1978; Santhanam, 1994; Macedo et al., 2001; Hansen, 2002) as well as to significantly enhance the surface water pH of areas as large as the North Sea (Brussaard et al., 1996).
In one recent example, Middelboe and Hansen (2007) studied the pH of a wave-exposed boulder reef in Aalsgaarde on the northern coast of Zealand, Denmark, and a sheltered shallow-water area in Kildebakkerne in the estuary Roskilde Fjord, Denmark, reporting that, in line with what one would expect if photosynthesis tends to increase surface-water pH, (1) "daytime pH was significantly higher in spring, summer and autumn than in winter at both study sites," often reaching values of 9 or more during peak summer growth periods vs. 8 or less in winter, that (2) "diurnal measurements at the most exposed site showed significantly higher pH during the day than during the night," reaching values that sometimes exceeded 9 during daylight hours but that typically dipped below 8 at night, and (3) that "diurnal variations were largest in the shallow water and decreased with increasing water depth."
In addition to their own findings, Middelboe and Hansen cite those of (1) Pearson et al. (1998), who found that pH averaged about 9 during the summer in populations of Fucus vesiculosus in the Baltic Sea, (2) Menendez et al. (2001), who found that maximum pH was 9 to 9.5 in dense floating macroalgae in a brackish coastal lagoon in the Ebro River Delta, and (3) Bjork et al. (2004), who found pH values as high as 9.8 to 10.1 in isolated rock pools in Sweden. Noting that "pH in the sea is usually considered to be stable at around 8 to 8.2," the two Danish researchers thus concluded that "pH is higher in natural shallow-water habitats than previously thought."
With each succeeding year, the physical evidence against the CO2-reduced calcification theory continues to grow ever more compelling, while support for the positive view promoted here continues to accumulate. Working in the laboratory, for example, Reynaud et al. (2004) grew nubbins of the branching zooxanthellate scleractinian coral Acropora verweyi in aquariums maintained at 20, 25 and 29°C, while weighing them once a week over a period of four weeks. This exercise revealed that coral calcification rates increased in nearly perfect linear fashion with increasing water temperature, yielding values of 0.06, 0.22 and 0.35% per day at 20, 25 and 29°C, respectively. These data reveal an approximate 480% increase in calcification rate in response to a 9°C increase in water temperature and a 160% increase in response to a 3°C increase in temperature, the latter of which temperature increases is somewhere in the low to midrange of global warming that climate alarmists claim will result from a 300 ppm increase in the air's CO2 concentration; and this positive temperature effect far outweighs the negative effect of rising CO2 concentrations on coral calcification via ocean acidification.
Working in the field, or, more correctly, the ocean, Carricart-Ganivet (2004) developed relationships between coral calcification rate and annual average SST based on data collected from colonies of the reef-building coral Montastraea annularis at twelve localities in the Gulf of Mexico and the Caribbean Sea, finding that calcification rate in the Gulf of Mexico increased 0.55 g cm-2 year-1 for each 1°C increase, while in the Caribbean Sea it increased 0.58 g cm-2 year-1 for each 1°C increase. Pooling these data with those of M. annularis and M. faveolata growing to a depth of 10 m at Carrie Bow Cay, Belize, those from reefs at St. Croix in the US Virgin Islands, and those of M. faveolata growing to a depth of 10 m at Curacao, Antilles, Carricart-Ganivet reports he obtained a mean increase in calcification rate of ~0.5 g cm-2 year-1 for each 1°C increase in annual average SST, which is even greater than what was found by Lough and Barnes for Porites corals.
In another important study, McNeil et al. (2004) used a coupled atmosphere-ice-ocean carbon cycle model to calculate annual mean SST increases within the world's current coral reef habitat from 1995 to 2100 for increases in the air's CO2 concentration specified by the IPCC's IS92a scenario, after which concomitant changes in coral reef calcification rates were estimated by combining the output of the climate model with empirical relationships between coral calcification rate and (1) aragonite saturation state (the negative CO2 effect) and (2) annual SST (the positive temperature effect). Their choice for the first of these two relationships was that derived by Langdon et al. (2000), which leads to an even greater reduction in calcification than was predicted in the study of Kleypas et al. Their choice for the second relationship was that derived by Lough and Barnes (2000), which leads to an increase in calcification that is only half as large as that derived by Carricart-Ganivet (2004). As a result, it can be appreciated that the net result of the two phenomena was doubly weighted in favor of reduced coral calcification. Nevertheless, McNeil et al. found that the increase in coral reef calcification associated with ocean warming far outweighed the decrease associated with the CO2-induced decrease in aragonite saturate state. In fact, they calculated that coral calcification in 2100 would be 35% higher than what it was in pre-industrial times at the very least. And, of course, they found that the area of coral reef habitat expands in association with the projected ocean warming.
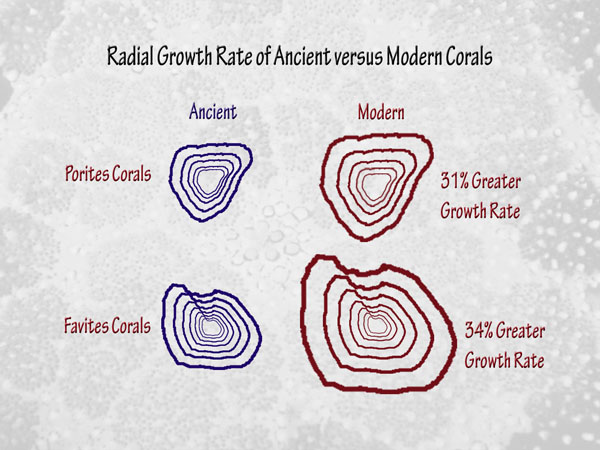
Finally, in a study devoted to corals that involves a much longer period of time than all of the others we have discussed, another research team (Crabbe et al., 2006) determined the original growth rates of long-dead Quaternary corals found in limestone deposits of islands in the Wakatobi Marine National Park of Indonesia, after which they compared them to the growth rates of present-day corals of the same genera living in the same area. This work revealed that the Quaternary corals grew “in a comparable environment to modern reefs” -- except, of course, for the air’s CO2 concentration, which is currently higher than it has been at any other time throughout the entire Quaternary, which spans the past 1.8 million years. Most interestingly, therefore, their measurements indicated that the radial growth rates of the modern corals were 31% greater than those of their ancient predecessors in the case of Porites species, and 34% greater in the case of Favites species.
To these papers we could add many others (Clausen and Roth, 1975; Coles and Jokiel, 1977; Kajiwara et al., 1995; Nie et al., 1997; Reynaud-Vaganay et al., 1999; Reynaud et al., 2007) that also depict increasing rates of coral calcification in the face of rising temperatures and atmospheric CO2 concentrations. Clearly, the net impact of 20th-century increases in atmospheric CO2 and temperature has not been anywhere near as catastrophically disruptive to earth’s corals as climate-alarmist dogma suggests it should have been. Quite to the contrary, the temperature and CO2 increases appear to not have been hurtful at all. In fact, they actually appear to have been helpful. Why? Because, as noted above, coral calcification is a biologically-driven process that can overcome physical-chemical limitations, which in the absence of life would appear to be insurmountable. But what about other calcifying and even non-calcifying marine organisms? Have they been harmed in any way?
1.3. Other Marine Organisms
In a paper recently published in Limnology and Oceanography, Richardson and Gibbons (2008) say there has been a drop of 0.1 pH unit in the global ocean since the start of the Industrial Revolution, and that "such acidification of the ocean may make calcification more difficult for calcareous organisms," resulting in the "opening [of] ecological space for non-calcifying species." In line with this thinking, they report that Attrill et al. (2007) have argued that "jellyfish may take advantage of the vacant niches made available by the negative effects of acidification on calcifying plankton," causing jellyfish to become more abundant; and they note that the latter researchers provided some evidence for this effect in the west-central North Sea over the period 1971-1995. Hence, they undertook a study to see if Attrill et al.'s findings (which were claimed to be the first of their kind) could be replicated on a much larger scale.
Working with data from a larger portion of the North Sea, as well as throughout most of the much vaster Northeast Atlantic Ocean, Richardson and Gibbons used coelenterate (jellyfish) records from the Continuous Plankton Recorder (CPR) and pH data from the International Council for the Exploration of the Sea (ICES) for the period 1946-2003 to explore the possibility of a relationship between jellyfish abundance and acidic ocean conditions. This work revealed that there were, as they describe it, "no significant relationships between jellyfish abundance and acidic conditions in any of the regions investigated."
In harmony with their findings, the two researchers note that "no observed declines in the abundance of calcifiers with lowering pH have yet been reported." In addition, they write that the "larvae of sea urchins form skeletal parts comprising magnesium-bearing calcite, which is 30 times more soluble than calcite without magnesium," and, therefore, that "lower ocean pH should drastically inhibit [our italics] the formation of these soluble calcite precursors." Yet they report that "there is no observable negative effect of pH." In fact, they say that echinoderm larvae in the North Sea have actually exhibited "a 10-fold increase [our italics] in recent times," which they say has been "linked predominantly to warming (Kirby et al., 2007)." Likewise, they further note that even in the most recent IPCC report, "there was no empirical evidence reported for the effect of acidification on marine biological systems (Rosenzweig et al., 2007)," in spite of all the concern that has been raised by acidification alarmists claiming that such is, or should be, occurring.
In light of this body of real-world evidence, or non-evidence, Richardson and Gibbons conclude (rather generously, we might add) that "the role of pH in structuring zooplankton communities in the North Sea and further afield at present is tenuous."
In another study, Vogt et al. (2008) examined the effects of atmospheric CO2 enrichment on various marine microorganisms in nine marine mesocosms in a fjord adjacent to the Large-Scale Facilities of the Biological Station of the University of Bergen in Espegrend, Norway. Three of the mesocosms were maintained at ambient levels of CO2 (~375 ppm), three were maintained at levels expected to prevail at the end of the current century (760 ppm or 2x CO2), and three were maintained at levels predicted for the middle of the next century (1150 ppm or 3x CO2), while measurements of numerous ecosystem parameters were made over a period of 24 days.
Results of the analysis showed no significant phytoplankton species shifts between treatments, and that "the ecosystem composition, bacterial and phytoplankton abundances and productivity, grazing rates and total grazer abundance and reproduction were not significantly affected by CO2 induced effects," citing in support of this statement the work of Riebesell et al. (2007), Riebesell et al. (2008), Egge et al. (2007), Paulino et al. (2007), Larsen et al. (2007), Suffrian et al. (2008) and Carotenuto et al. (2007). With respect to their many findings, the eight researchers say their observations suggest that "the system under study was surprisingly resilient to abrupt and large pH changes," which is just the opposite of what the world's acidification alarmists characteristically predict about CO2-induced "ocean acidification."
Expanding the subject of CO2 effects on other marine organisms, Gutowska et al. (2008) studied the cephalopod mollusk Sepia officinalis and found that it “is capable of not only maintaining calcification, but also growth rates and metabolism when exposed to elevated partial pressures of carbon dioxide.” Over a six-week test period, for example, they found that “juvenile S. officinalis maintained calcification under ~4000 and ~6000 ppm CO2, and grew at the same rate with the same gross growth efficiency as did control animals,” gaining approximately 4% body mass daily and increasing the mass of their calcified cuttlebone by over 500%. These findings thus led them to specifically conclude that “active cephalopods possess a certain level of pre-adaptation to long-term increments in carbon dioxide levels,” and to generally conclude that our “understanding of the mechanistic processes that limit calcification must improve before we can begin to predict what effects future ocean acidification will have on calcifying marine invertebrates.”
In another study, Berge et al. (2006) continuously supplied five 5-liter aquariums with low-food-content sea water that was extracted from the top meter of the Oslofjord outside the Marine Research Station Solbergstrand in Norway, while CO2 was continuously added to the waters of the aquaria so as to maintain them at five different pH values (means of 8.1, 7.6, 7.4, 7.1 and 6.7) for a period of 44 days. Prior to the start of the study, blue mussels (Mytilus edulis) of two different size classes (mean lengths of either 11 or 21 mm) were collected from the outer part of the Oslofjord, and 50 of each size class were introduced into each aquarium, where they were examined close to daily for any deaths that may have occurred, after which shell lengths at either the time of death or at the end of the study were determined and compared to lengths measured at the start of the study. Simultaneously, water temperature rose slowly from 16 to 19°C during the initial 23 days of the experiment, but then declined slightly to day 31, after which it rose rapidly to attain a maximum value of 24°C on day 39.
A lack of mortality during the first 23 days of the study showed, in the words of the researchers, that "the increased concentration of CO2 in the water and the correspondingly reduced pH had no acute effects on the mussels." Thereafter, however, some mortality was observed in the highest CO2 (lowest pH) treatment from day 23 to day 37, after which deaths could also be observed in some of the other treatments, which mortality Berge et al. attributed to the rapid increase in water temperature that occurred between days 31 and 39.
With respect to growth, the Norwegian researchers report that "mean increments of shell length were much lower for the two largest CO2 additions compared to the values in the controls, while for the two smallest doses the growth [was] about the same as in the control, or in one case even higher (small shells at pH = 7.6)," such that there were "no significant differences between the three aquaria within the pH range 7.4-8.1."
Berge et al. say their results "indicate that future reductions in pH caused by increased concentrations of anthropogenic CO2 in the sea may have an impact on blue mussels," but that "comparison of estimates of future pH reduction in the sea (Caldeira and Wickett, 2003) and the observed threshold for negative effects on growth of blue mussels [which they determined to lie somewhere between a pH of 7.4 and 7.1] do however indicate that this will probably not happen in this century." Indeed, Caldeira and Wickett's calculation of the maximum level to which the air's CO2 concentration might rise yields a value that approaches 2000 ppm around the year 2300, representing a surface oceanic pH reduction of 0.7 units, which only drops the pH to the upper limit of the "threshold for negative effects on growth of blue mussels" found by Berge et al., i.e., 7.4. Consequently, blue mussels will likely never be bothered, even in the least degree, by the tendency for atmospheric CO2 enrichment to lower oceanic pH values.
In a study of a very different creature, Langer et al. (2006) conducted batch-culture experiments on two coccolithophores, Calcidiscus leptoporus and Coccolithus pelagicus, in which they observed a "deterioration of coccolith production above as well as below present-day CO2 concentrations in C. leptoporus," and a "lack of a CO2 sensitivity of calcification in C. pelagicus" over an atmospheric CO2 concentration range of 98-915 ppm. Both of these observations, in their words, "refute the notion of a linear relationship of calcification with the carbonate ion concentration and carbonate saturation state," which refuted notion is championed by the world's climate alarmists. In an apparent negative finding, however, particularly in the case of C. leptoporus, Langer et al. observed that although their experiments revealed that "at 360 ppm CO2 most coccoliths show normal morphology," at both "higher and lower CO2 concentrations the proportion of coccoliths showing incomplete growth and malformation increases notably."
To determine if such deleterious responses might have also occurred in the real world at different times in the past, the researchers studied coccolith morphologies in six sediment cores obtained along a range of latitudes in the Atlantic Ocean. As they describe it, this work revealed that changes in coccolith morphology similar to those "occurring in response to the abrupt CO2 perturbation applied in experimental treatments are not [our italics] mirrored in the sedimentary record." This finding indicates, as they suggest, that "in the natural environment C. leptoporus has adjusted to the 80-ppm CO2 and 180-ppm CO2 difference between present [and] preindustrial and glacial times, respectively."
In further discussing these observations, Langer et al. say "it is reasonable to assume that C. leptoporus has adapted its calcification mechanism to the change in carbonate chemistry having occurred since the last glacial maximum," suggesting as a possible explanation for this phenomenon that "the population is genetically diverse, containing strains with diverse physiological and genetic traits, as already demonstrated for E. huxleyi (Brand, 1981, 1982, 1984; Conte et al., 1998; Medlin et al., 1996; Paasche, 2002; Stolte et al., 2000)." They also state that this adaptive ability "is not likely to be confined to C. leptoporus but can be assumed to play a role in other coccolithophore species as well," which leads them to conclude that such populations "may be able to evolve so that the optimal CO2 level for calcification of the species tracks the environmental value [our italics]." With respect to the future, therefore, Langer et al. end on a strongly positive note, stating that "genetic diversity, both between and within species, may allow calcifying organisms to prevail in a high CO2 ocean."
 Focusing on another coccolithophore species, Riebesell (2004) notes that "a moderate increase in CO2 facilitates photosynthetic carbon fixation of some phytoplankton groups," including "the coccolithophorids Emiliania huxleyi and Gephyrocapsa oceanica." Hence, in a major challenge to the climate-alarmist claim that atmospheric CO2 enrichment will definitely harm such marine organisms, Riebesell suggests that "CO2-sensitive taxa, such as the calcifying coccolithophorids, should therefore benefit more [our italics] from the present increase in atmospheric CO2 compared to the non-calcifying diatoms."
Focusing on another coccolithophore species, Riebesell (2004) notes that "a moderate increase in CO2 facilitates photosynthetic carbon fixation of some phytoplankton groups," including "the coccolithophorids Emiliania huxleyi and Gephyrocapsa oceanica." Hence, in a major challenge to the climate-alarmist claim that atmospheric CO2 enrichment will definitely harm such marine organisms, Riebesell suggests that "CO2-sensitive taxa, such as the calcifying coccolithophorids, should therefore benefit more [our italics] from the present increase in atmospheric CO2 compared to the non-calcifying diatoms."
In support of this suggestion, Riebesell describes the results of some CO2 perturbation experiments conducted south of Bergen, Norway, where nine 11-m3 enclosures moored to a floating raft were aerated in triplicate with CO2-depleted, normal and CO2-enriched air to achieve CO2 levels of 190, 370 and 710 ppm, simulating glacial, present-day and predicted conditions for the end of the century, respectively. In the course of the study, a bloom consisting of a mixed phytoplankton community developed, and, in Riebesell's words, "significantly higher net community production was observed under elevated CO2 levels during the build-up of the bloom." He further reports that "CO2-related differences in primary production continued after nutrient exhaustion, leading to higher production of transparent exopolymer particles under high CO2 conditions," something that has also been observed by Engel (2002) in a natural plankton assemblage and by Heemann (2002) in monospecific cultures of both diatoms and coccolithophores.
Another important finding of this experiment was that the community that developed under the high CO2 conditions expected for the end of this century was dominated by Emiliania huxleyi. Consequently, Riebesell finds even more reason to believe that "coccolithophores may benefit from the present increase in atmospheric CO2 and related changes in seawater carbonate chemistry," in contrast to the many negative predictions that have been made about rising atmospheric CO2 concentrations in this regard. Finally, in further commentary on the topic, Riebesell states that "increasing CO2 availability may improve the overall resource utilization of E. huxleyi and possibly of other fast-growing coccolithophore species," concluding that "if this provides an ecological advantage for coccolithophores, rising atmospheric CO2 could potentially increase the contribution of calcifying phytoplankton to overall primary production." In fact, noting that "a moderate increase in CO2 facilitates photosynthetic carbon fixation of some phytoplankton groups," including "the coccolithophorids Emiliania huxleyi and Gephyrocapsa oceanica" - and in a major challenge to the climate-alarmist claim that atmospheric CO2 enrichment will harm such marine organisms - Riebesell suggests that "CO2-sensitive taxa, such as the calcifying coccolithophorids, should therefore benefit more [our italics] from the present increase in atmospheric CO2 compared to the non-calcifying diatoms."
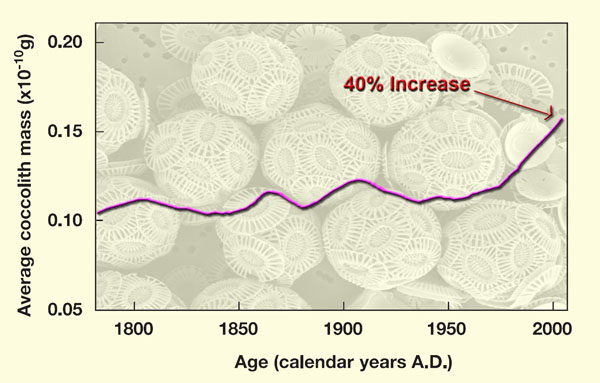
Support of Riebesell's findings was recently provided by an international team of thirteen researchers (Iglesias-Rodriguez et al., 2008), who bubbled air of a number of different atmospheric CO2 concentrations through culture media containing the phytoplanktonic coccolithophore species Emiliania hyxleyi, while determining the amounts of particulate organic and inorganic carbon they produced. In addition, they determined the real-world change in average coccolithophore mass over the past 220 years in the subpolar North Atlantic Ocean, based on data obtained from a sediment core, over which period of time the atmosphere’s CO2 concentration rose by approximately 90 ppm and the earth emerged from the frigid depths of the Little Ice Age to experience the supposedly unprecedented high temperatures of the Current Warm Period.
Results of their analysis revealed an approximate doubling of both particulate organic and inorganic carbon between the culture media in equilibrium with air of today’s CO2 concentration and the culture media in equilibrium with air of 750 ppm CO2. In addition, they say the field evidence they obtained from the deep-ocean sediment core they studied “is consistent with these laboratory conclusions,” and that it indicates that “over the past 220 years there has been a 40% increase in average coccolith mass.”
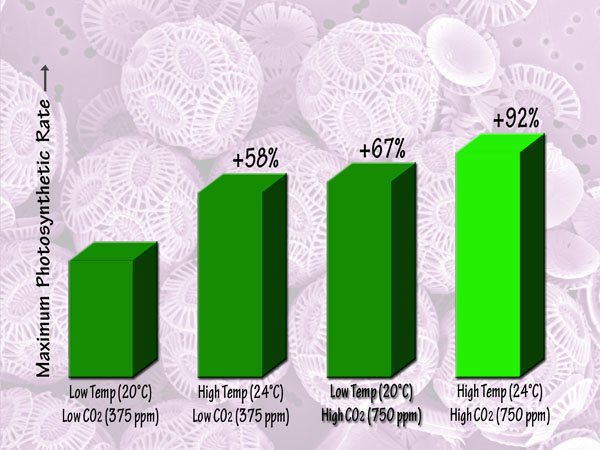
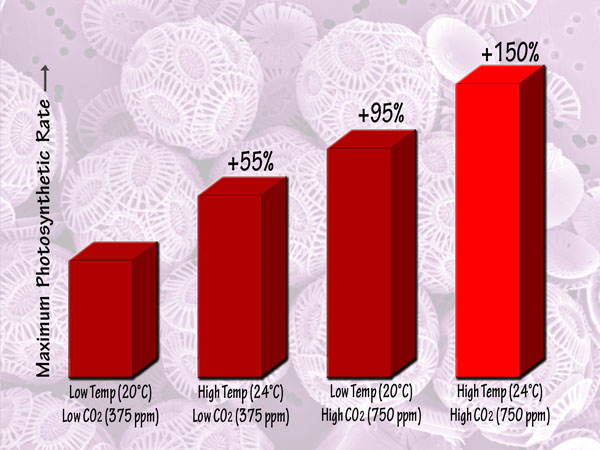 Focusing more on the future, a third independent team of seven scientists (Feng et al., 2008) studied Emiliania huxleyi coccoliths that they isolated from the Sargasso Sea, and which they grew in semi-continuous culture media at low and high light intensities, low and high temperatures (20 and 24°C), and low and high CO2 concentrations (375 and 750 ppm). This work revealed that in the low-light environment, the maximum photosynthetic rate was lowest in the low-temperature, low-CO2 or ambient treatment, but was increased by 55% by elevated temperature alone and by 95% by elevated CO2 alone, while in the high-temperature, high-CO2 or greenhouse treatment it was increased by 150% relative to the ambient treatment. Likewise, in the high-light environment, there were maximum photosynthetic rate increases of 58%, 67% and 92% for the elevated temperature alone, elevated CO2 alone and greenhouse treatments, respectively. Consequently, the researchers concluded, in their words, that “future trends of CO2 enrichment, sea-surface warming and exposure to higher mean irradiances from intensified stratification will have a large influence on the growth of Emiliania huxleyi.” And, of course, that “large influence” will be positive, and tremendously so.
Focusing more on the future, a third independent team of seven scientists (Feng et al., 2008) studied Emiliania huxleyi coccoliths that they isolated from the Sargasso Sea, and which they grew in semi-continuous culture media at low and high light intensities, low and high temperatures (20 and 24°C), and low and high CO2 concentrations (375 and 750 ppm). This work revealed that in the low-light environment, the maximum photosynthetic rate was lowest in the low-temperature, low-CO2 or ambient treatment, but was increased by 55% by elevated temperature alone and by 95% by elevated CO2 alone, while in the high-temperature, high-CO2 or greenhouse treatment it was increased by 150% relative to the ambient treatment. Likewise, in the high-light environment, there were maximum photosynthetic rate increases of 58%, 67% and 92% for the elevated temperature alone, elevated CO2 alone and greenhouse treatments, respectively. Consequently, the researchers concluded, in their words, that “future trends of CO2 enrichment, sea-surface warming and exposure to higher mean irradiances from intensified stratification will have a large influence on the growth of Emiliania huxleyi.” And, of course, that “large influence” will be positive, and tremendously so.
Clearly, climate-alarmist claims of impending marine species extinctions due to increases in both temperature and atmospheric CO2 concentration are not only not supported by real-world evidence, they are actually refuted by it.




