The planting and preservation of forests has long been acknowledged to be an effective and environmentally-friendly means for slowing climate-model-predicted CO2-induced global warming. This prescription for moderating potential climate change is based on two well-established and very straightforward facts: (1) the carbon trees use to construct their tissues comes from the air, and (2) its extraction from the atmosphere slows the rate of rise of the air's CO2 content.
Although simple enough that a child can understand it, this potential partial solution to the putative global warming problem has been under attack for several years by people who seek to address the issue solely on the basis of forced reductions in anthropogenic CO2 emissions (see Pearce, 1999). The tack they take in this campaign is to claim that carbon sequestration by forests is only viable when forests are young and growing vigorously; for as forests age, according to the regulatory-minded pundits, they gradually lose their carbon sequestering prowess, such that forests more than one hundred years old become essentially useless for removing CO2 from the air, as they claim that such old and decrepit stands yearly lose as much CO2 via respiration as what they acquire via photosynthesis.
As with all things, thankfully, real-world data typically tell the truth. So what do we learn about the matter from actual measurements made on older real-world trees?
In Panama (Condit et al., 1995), Brazil (Chambers et al., 1998; Laurance et al., 2004; Chambers et al., 2001), and many parts of the southwestern United States (Graybill and Idso, 1993), individuals of a number of different species have been shown to live for nearly one and a half millennia. At a hundred or so years of age, these super-slurpers of CO2 are mere youngsters. And in their really old age, their appetite for the vital gas, though diminished, is not lost. In fact, Chambers et al. (1998) indicated that the long-lived trees of Brazil continue to experience protracted slow growth even at 1400 years of age. And the protracted slow growth (evident in yearly increasing trunk diameters) of very old and large trees can absorb a huge amount of CO2 out of the air each year, especially when - as noted by Chanbers et al. (1998) with respect to the Brazilian forests in the central Amazon - about 50% of their above-ground biomass is contained in less than the largest 10% of their trees. Consequently, since the life span of these massive long-lived trees is considerably greater than the projected life span of the entire "Age of Fossil Fuels," their cultivation and preservation represents an essentially permanent partial solution to the perceived problem of the dreaded global warming that climate alarmists ascribe to anthropogenic CO2 emissions.
As important as are these facts about trees, however, there's an even more important fact that comes into play in the case of forests and their ability to sequester carbon over long periods of time. This little-acknowledged piece of information is the fact that it is the forest itself - conceptualized as a huge super-organism, if you will - that is the unit of primary importance when it comes to determining the ultimate amount of carbon that can be sequestered on a unit area of land. And it when it comes to elucidating this concept, it seems that a lot of climate alarmists and political opportunists can't seem to see the forest for the trees that comprise it.
That this difference in perspective can have enormous consequences was demonstrated quite clearly by Cary et al. (2001), who noted that most models of forest carbon sequestration wrongly assume that "age-related growth trends of individual trees and even-aged, monospecific stands can be extended to natural forests." When they compared the predictions of such models against real-world data gathered from northern Rocky Mountain subalpine forests that ranged in age from 67 to 458 years, for example, they found that aboveground net primary productivity in 200-year-old natural stands was almost twice as great as that of modeled stands, and that the difference between the two increased linearly throughout the entire sampled age range.
So what was the explanation for the huge discrepancy? Cary et al. suggested that long-term recruitment and the periodic appearance of additional late-successional species (increasing biodiversity) may have significant effects on stand productivity, infusing the primary unit of concern (i.e., the ever-evolving forest super-organism) with greater vitality than would have been projected on the basis of characteristics possessed by the unit earlier in its life. They also noted that by not including effects of size- or age-dependent decreases in stem and branch respiration per unit of sapwood volume in models of forest growth, respiration in older stands can be over-estimated by a factor of two to five.
How serious are these model shortcomings? For the real-world forests studied by Cary et al., they produced predictions of carbon sequestration that were only a little over half as large as what was observed in nature for 200-year-old forests; while for 400-year-old forests, they produced results that were only about a third as large as what was characteristic of the real world. And as the forests grew older still, the difference between reality and model projections grew right along with them.
Another study about the suitability of forests to act as long-term carbon sinks was conducted by Lou et al. (2003), who analyzed data obtained from the Duke Forest FACE experiment, in which three 30-meter-diamerer plots within a 13-year old forest (composed primarily of loblolly pines with sweetgum and yellow poplar trees as sub-dominants, together with numerous other trees, shrubs and vines that occupied still smaller niches) began to be enriched with an extra 200 ppm of CO2 in August of 1996, while three similar plots were maintained at the ambient atmospheric CO2 concentration. A number of papers describing different facets of this long-term study have been published; and as recounted by Lou et al., they have revealed the existence of a CO2-induced "sustained photosynthetic stimulation at leaf and canopy levels [Myers et al., 1999; Ellsworth, 2000; Luo et al., 2001; Lai et al., 2002], which has resulted in sustained stimulation of wood biomass increment [Hamilton et al., 2002] and a larger carbon accumulation in the forest floor at elevated CO2 than at ambient CO2 [Schlesinger and Lichter, 2001]."
Based upon these findings and what they implied about rates of carbon removal from the atmosphere and its different residence times in plant, litter and soil carbon pools, Luo et al. developed a model for studying the sustainability of forest carbon sequestration. Applying this model to a situation where the atmospheric CO2 concentration gradually rises from a value of 378 ppm in 2000 to a value of 710 ppm in 2100, they calculated that the carbon sequestration rate of the Duke Forest would rise from an initial value of 69 g m-2 yr-1 to a final value of 201 g m-2 yr-1, which is a far, far cry from the sad scenario promulgated by the cadre of climate alarmists that have long claimed that earth's forests will have released much of the carbon they had previously absorbed as early as the year 2050 (Pearce, 1999).
Another study that supports the long-term viability of carbon sequestration by forests was conducted by Paw U et al. (2004), who also noted that old-growth forests have generally been considered to "represent carbon sources or are neutral (Odum, 1963, 1965)," stating that "it is generally assumed that forests reach maximum productivity at an intermediate age and productivity declines in mature and old-growth stands (Franklin, 1988), presumably as dead woody debris and other respiratory demands increase." More particularly, they reported that a number of articles have suggested that "old-growth conifer forests are at equilibrium with respect to net ecosystem productivity or net ecosystem exchange (DeBell and Franklin, 1987; Franklin and DeBell, 1988; Schulze et al., 1999), as an age-class end point of ecosystem development."
To see if these claims had any merit, Paw U et al. used an eddy covariance technique to estimate the CO2 exchange rate of the oldest forest ecosystem (500 years old) in the AmeriFlux network of carbon-flux measurement stations - the Wind River old-growth forest in southwestern Washington, USA, which is composed mainly of Douglas-fir and western Hemlock - over a period of 16 months, from May 1998 to August 1999. Throughout this period, the fourteen scientists reported "there were no monthly averages with net release of CO2," and that the cumulative net ecosystem exchange showed "remarkable sequestration of carbon, comparable to many younger forests." Hence, they concluded that "in contrast to frequently stated opinions, old-growth forests can be significant carbon sinks," noting that "the old-growth forests of the Pacific Northwest can contribute to optimizing carbon sequestration strategies while continuing to provide ecosystem services essential to supporting biodiversity."
Yet another study to ask and address the question "Do old forests gain or lose carbon?" was that of Binkley et al. (2004), who revisited an aging aspen forest in the Tesuque watershed of northern New Mexico, USA - which between 1971 and 1976 (when it was between 90 and 96 years old) was thought to have had a negative net ecosystem production rate of -2.0 Mg ha-1 yr-1 - and measured the basal diameters of all trees in the central 0.01 ha of each of 27 plots arrayed across the watershed, after which they used the same regression equations employed in the earlier study to calculate live tree biomass as of 2003.
"Contrary to expectation," as they described it, Binkley et al. found that "live tree mass in 2003 [186 Mg ha-1] was significantly greater than in 1976 [149 Mg ha-1] (P = 0.02), refuting the hypothesis that live tree mass declined." In fact, they found that the annual net increment of live tree mass was about 1.37 Mg ha-1 yr-1 from age 96 to age 123 years, which is only 12% less than the mean annual increment of live tree mass experienced over the forest's initial 96 years of existence (149 Mg ha-1 / 96 yr = 1.55 Mg ha-1 yr-1). Consequently, in response to the question they posed when embarking on their study - "Do old forests gain or lose carbon?" - Binkley et al. concluded that "old aspen forests continue to accrue live stem mass well into their second century, despite declining current annual increments," which, we might add, are not all that much smaller than those the forests exhibited in their younger years.
Similar results were obtained by Hollinger et al. (1994) for a 300-year-old Nothofagus site in New Zealand, by Law et al. (2001) for a 250-year-old ponderosa pine site in the northwestern United States, by Falk et al. (2002) for a 450-year-old Douglas fir/western hemlock site in the same general area, and by Knohl et al. (2003) for a 250-year-old deciduous forest in Germany. And in commenting on these findings, the latter investigators said they found "unexpectedly high carbon uptake rates during two years for an unmanaged 'advanced' beech forest, which is in contrast to the widely spread hypothesis that 'advanced' forests are insignificant as carbon sinks." Thus, for the forest they studied, as they described it, "assimilation is clearly not balanced by respiration, although this site shows typical characteristics of an 'advanced' forest at a comparatively late stage of development."
These observations about forests are remarkably similar to recent findings regarding humans, i.e., that non-genetic interventions, even late in life, can put one on a healthier trajectory that extends productive lifespan. So what is the global "intervention" that has put the planet's trees on the healthier trajectory of being able to sequester significant amounts of carbon in their old age, when past theory (which was obviously based on past observations) decreed that they should be in a state of no-net-growth or even negative growth?
The answer is rather simple. For any tree of age 250 years or more, the greater portion of its life (at least two-thirds of it) was spent in an atmosphere of much-reduced CO2 content. Up until 1920, for example, the air's CO2 concentration had never been above 300 ppm throughout the entire lives of such trees, whereas it is currently 400 ppm or 33% higher. And for older trees, even greater portions of their lives were spent in air of even lower CO2 concentration. Hence, the "intervention" that has given new life to old trees and allows them to "live long and prosper," would appear to be the aerial fertilization effect produced by the flooding of the air with the CO2 that resulted from the Industrial Revolution and that is currently being maintained by its ever-expanding aftermath (Idso, 1995).
Based on these many observations, as well as the results of the study of Greenep et al. (2003) - which strongly suggested, in their words, that "the capacity for enhanced photosynthesis in trees growing in elevated CO2 is unlikely to be lost in subsequent generations" - it would appear that earth's forests will remain strong sinks for atmospheric carbon far beyond the date at which the world's climate alarmists have proclaimed they would have given back to the atmosphere most of the carbon they had removed from it over their existence to that point in time. And subsequent reports have validated this assessment.
Forging forward, for example, Zhou et al. (2006) noted that "old-growth forests have traditionally been considered negligible as carbon sinks because carbon uptake has been thought to be balanced by respiration." And as a result, they reported that "the soil carbon balance of old-growth forests has received little attention." So in an attempt to rectify this situation, they conducted a study to measure the long-term (1979 to 2003) dynamics of soil organic carbon stock in old-growth forests (age > 400 years) at the Dinghushan Biosphere Reserve in Guangdong Province, China." In doing so, the eight scientists determined that "soil organic carbon concentration in the top 20-cm soil layer increased between 1979 and 2003 from about 1.4% to 2.35% at an average rate of 0.035% each year," and that "measurements on a total of 230 composite soil samples collected between 1979 and 2003 suggested that soil organic carbon stock in the top 20-cm soil layer increased significantly during that time (P < 0.0001), with an average rate of 0.61 Mg C ha-1 year-1." And in discussing their results, Zhou et al. stated that although "the driving forces for this observed high rate of soil organic carbon increase in the old-growth forests are not clear at present," their study "suggests that the carbon cycle processes in the belowground system of these forests are changing in response to the changing environment."
Two years later, Luyssaert et al. (2008) conducted a literature survey to test the hypothesis that forests continue to acquire and sequester carbon from the atmosphere for literally hundreds of years, compiling data from 519 plot studies conducted throughout the world's boreal and temperate forests (30% and 70% of the studies, respectively), but skipping the tropics because of the low number of tropical sites that possessed the net ecosystem production (NEP) and forest age estimates needed for their analysis. In doing so, they determined that "in forests between 15 and 800 years old, the NEP is usually positive; that is, the forests are CO2 sinks." In fact, they found that "young forests rather than old-growth forests are very often conspicuous sources of CO2 because the creation of new forests (whether naturally or by humans) frequently follows disturbance to soil and the previous vegetation, resulting in a decomposition rate of coarse woody debris, litter and soil organic matter that exceeds the net primary production of the regrowth." And in discussing the implications of their findings, the team of American, Belgian, British, French, German and Swiss researchers wrote that (1) "because old-growth forests steadily accumulate carbon for centuries, they contain vast quantities of it," and that (2) "they will lose much of this carbon to the atmosphere if they are disturbed, so carbon-accounting rules for forests should give credit for leaving old-growth forests intact," thereby letting them continue to sequester even more carbon.
About this same time, Phillips et al. (2008) published a study in which they said there was "a long held view," as they described it, that "old trees exhibit little potential for growth." And they therefore stated that "it may seem reasonable to conclude that old trees are not responsive to increased CO2," as many climate alarmists had indeed claimed. They went on, however, to demonstrate that this view was far from the truth.
The three researchers begin their analysis of the subject by stating that "hydraulic constraints in tall trees," such as those of great age, "constitute a fundamental form of water limitation; indeed, one that is indistinguishable from soil water limitations," citing the work of Koch et al. (2004) and Woodruff et al. (2004). They also reported that "recent research indicates that tree size and its hydraulic correlates, rather than age per se, controls carbon gain in old trees," as indicated by the study of Mencuccini et al. (2005). These findings implied, in their words, that "factors that alleviate internal or external resource constraints on old trees could improve physiological function and ultimately growth," which is something elevated CO2 does quite well by increasing plant water use efficiency. In fact, they listed several phenomena that suggest "a fundamental potential for old growth trees to show greater photosynthesis and growth under industrial age increases in CO2 than they would under constant, pre-industrial CO2 levels."
Drawing from their own work, for example, Phillips et al. reported that "500- and 20-year-old Douglas-fir trees both show high sensitivity of photosynthesis to atmospheric CO2," presenting data which clearly demonstrated, as they phrased it, that "under optimal conditions there exists the potential for an approximately 30% increase in photosynthetic rate with an increase in CO2 from pre-industrial to current levels [i.e., from 280 to 385 ppm] in old trees." And they went on to note that "the phenomenon of twentieth-century ring-width increase," which could thus be expected to accompany the 20th-century increase in the air's CO2 concentration, had in fact been detected in several other studies, including those of LaMarche et al., (1984), Jacoby (1986), Graybill (1987), Kienast and Luxmoore (1988), Graumlich (1991), Knapp et al. (2001), Bunn et al. (2005), and Soule and Knapp (2006), to which could also have been added Graybill and Idso (1993).
Just one year later, Phillips et al. (2009) wrote that old growth forests in Amazonia - through photosynthesis and respiration - process 18 petagrams [18 x 1015 grams] of carbon annually, which they said was "more than twice the rate of anthropogenic fossil fuel emissions." They also stated that over the past quarter-century of intensive region-wide measurements, the productivity of the Amazon rainforest - even in its extreme old age - had been found to be "increasing with time," in support of which statement they cited the comprehensive observational studies of Phillips et al. (1998), Nemani et al. (2003), Baker et al. (2004), Lewis et al. (2004) and Ichii et al. (2005).
Against the backdrop of this very positive phenomenon, the goal of Phillips et al.'s new analysis was to determine what negative effect a severe drought might possibly have on South America's surprisingly-spry-for-its-age tropical mega-forest, especially a drought of the type that the world's climate alarmists predict will occur if anthropogenic CO2 emissions are not significantly abated. What the international team of scientists wanted to know, essentially, was whether such a decline in the availability of water might wipe out the super ecosystem's biomass gains of prior decades, thereby fulfilling one of the climate alarmists' worst-case catastrophic scenarios.
Focusing their attention on the Amazonian drought of 2005, which they described as "one of the most intense droughts of the past 100 years," as well as "a possible analog of future events," the 66 researchers (who had monitored a host of forest plots across the Amazon basin over the prior quarter-century) utilized tree diameter, wood density, and allometric models to compute the basin's woody biomass at each time of measurement, both before and after the drought, deriving the results that are plotted in the following figure.
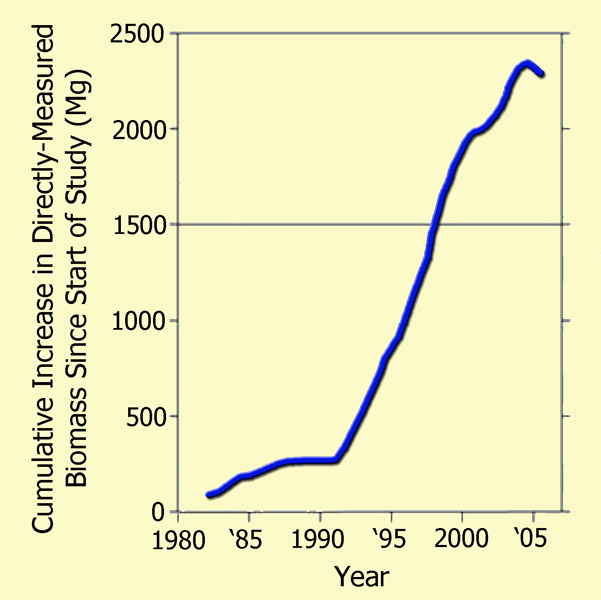
The post-1980 cumulative biomass increase of Amazon trees >= 10 cm in diameter as a function of the mid-date of each forest-plot census interval, portrayed as a 50-interval moving mean. Adapted from Phillips et al. (2009).
As may readily be seen from these real-world measurement-based results, the great Amazonian drought of 2005 resulted in only a slight hiatus in the strong upward trend of tree biomass accumulation that was exhibited over the prior two decades, which had occurred, as Phillips et al. noted, through a multi-decadal period spanning both wet and dry conditions, the latter of which are not even detectable in their wood biomass data. Hence, it would appear that although extremely severe drought conditions can indeed bring a temporary halt to biomass accumulation in old growth tropical forests - and sometimes even lead to minor reductions in biomass due to selective tree mortality - the vast majority of the trees are able to regain their photosynthetic prowess and add to their prior store of biomass once the moisture stress subsides, thanks in large measure to the enhanced growth (Lin et al., 1998) and water use efficiency (Hietz et al., 2005) that are experienced by nearly all woody plants as the air's CO2 content rises.
Additional support for this attribution is provided by the work of Lloyd and Farquhar (2008), who concluded that "the magnitude and pattern of increases in forest dynamics across Amazonia observed over the last few decades are consistent with a CO2-induced stimulation of tree growth," while still more support for the premise has come from the work of Phillips et al. (2008), who concluded that the simplest explanation for the phenomenon is that "improved resource availability has increased net primary productivity, in turn increasing growth rates," and who further noted that "the only change for which there is unambiguous evidence that the driver has widely changed and that such a change should accelerate forest growth is the increase in atmospheric CO2," because of "the undisputed long-term increase in [its] concentration, the key role of CO2 in photosynthesis, and the demonstrated effects of CO2 fertilization on plant growth rates."
Further support for this world-view came from old-growth forests of tropical Africa, where Lewis et al. (2009) invested a great amount of time and effort in documenting changes in aboveground carbon storage in 79 permanent plots spanning 40 years (1968-2007), located in closed-canopy moist forest, spanning West, Central and Eastern Africa, based on data obtained from more than 70,000 individual trees spread across ten countries. This work revealed, as they described it, that "aboveground carbon storage in live trees increased by 0.63 Mg C ha-1 year-1 between 1968 and 2007," and that "extrapolation to unmeasured forest components (live roots, small trees, necromass) and scaling to the continent implies a total increase in carbon storage in African tropical forest trees of 0.34 Pg C year-1."
In discussing these results, the 33 researchers stated that the observed changes in carbon storage were "similar to those reported for Amazonian forests per unit area, providing evidence that increasing carbon storage in old-growth forests is a pan-tropical phenomenon." They also said that "combining all standardized inventory data from this study and from tropical America and Asia together yields a comparable figure of 0.49 Mg C ha-1 year-1," which equates to "a carbon sink of 1.3 Pg C year-1 across all tropical forests during recent decades," which can account for roughly half of the global missing carbon sink. As for what the driving force was that seems to have breathed new life into tropical Africa's old trees, Lewis et al. wrote - in the concluding sentence of the abstract of their paper - that "taxon-specific analyses of African inventory and other data suggest that widespread changes in resource availability, such as increasing atmospheric carbon dioxide concentrations, may be the cause of the increase in carbon stocks, as some theory (Lloyd and Farquhar, 1996) and models (Friedlingstein et al., 2006; Stephens et al., 2007; Ciais et al., 2008) predict."
Last of all, Tan et al. (2011) introduced their thoughts on the subject by reporting the fact that stands of trees with ages in excess of 200 years have been demonstrated by several research groups to act as carbon sinks in both coniferous and mixed forests, citing the work of Hollinger et al. (1994), Law et al. (2001), Roser et al. (2002), Knohl et al. (2003), Paw et al. (2004), Desai et al. (2005) and Guan et al. (2006). And they went on to buttress this claim by reporting the results of their own study of the subject, in which they employed an eddy covariance technique to examine the carbon balance of a more-than-300-year-old subtropical evergreen broadleaved forest located in the center of the largest subtropical land area of the world in the Ailao Mountain Nature Reserve (24°32'N, 101°01'E) of Yunnan Province in Southwest China.
There, in addition to their micrometeorologically-based eddy flux carbon budget estimation, the six scientists conducted a tree inventory of one hectare of forest located within the footprint of the eddy flux tower they employed in November of 2003 and again in November of 2007, after which they compared measurements of tree diameter at breast height (DBH) between the two times and employed site specific allometric equations to derive mean yearly biomass production from the measurements obtained at the two times, while they also assessed aboveground litter production via the amount captured each year in 25 litter traps that were randomly distributed within the one-hectare plot.
As a result of their efforts, Tan et al. determined that the mean annual net ecosystem production of the forest was approximately 9 tC/ha/year, which suggested, in their words, that "this forest acts as a large carbon sink." In addition, their inventory data indicated that about 6 tC/ha/year was contributed by biomass and necromass. And they reported that approximately 60% of the biomass increment was contributed by the growth of large trees with breast height diameters in excess of 60 cm.
Clearly, therefore, the old notion of old trees contributing next to nothing to global carbon sequestration is manifestly invalid. They are ever hard at work, doing what they do best, sucking CO2 out of the air, and growing!
References
Baker, T.R., Phillips, O.L., Malhi, Y., Almeida, S., Arroyo, L., Di Fiore, A., Erwin, T., Higuchi, N., Killeen, T.J., Laurance, S.G., Laurance, W.F., Lewis, S.L., Monteagudo, A., Neill, D.A., Núñez Vargas, P., Pitman, N.C.A., Silva, J.N.M. and Vásquez Martínez, R. 2004. Increasing biomass in Amazonian forest plots. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 353-365.
Binkley, D., White, C.S. and Gosz, J.R. 2004. Tree biomass and net increment in an old aspen forest in New Mexico. Forest Ecology and Management 203: 407-410.
Bunn, A.G., Graumlich, L.J. and Urban, D.L. 2005. Trends in twentieth-century tree growth at high elevations in the Sierra Nevada and White Mountains, USA. The Holocene 15: 481-488.
Carey, E.V., Sala, A., Keane, R. and Callaway, R.M. 2001. Are old forests underestimated as global carbon sinks? Global Change Biology 7: 339-344.
Chambers, J.Q., Higuchi, N. and Schimel, J.P. 1998. Ancient trees in Amazonia. Nature 391: 135-136.
Chambers, J.Q., Van Eldik, T., Southon, J., Higuchi, N. 2001. Tree age structure in tropical forests of central Amazonia. In: Bierregaard, R.O., Gascon, C., Lovejoy, T., and Mesquita, R. (Eds.). Lessons from Amazonia: Ecology and Conservation of a Fragmented Forest. Yale University Press, New Haven, CT, USA, pp. 68-78.
Ciais, P., Piao, S.-L., Cadule, P., Friedlingstein, P. and Chedin, A. 2008. Variability and recent trends in the African carbon balance. Biogeosciences 5: 3497-3532.
Condit, R., Hubbell, S.P. and Foster, R.B. 1995. Mortality-rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecological Monographs 65: 419-439.
DeBell, D.S. and Franklin, J.S. 1987. Old-growth Douglas-fir and western hemlock: a 36-year record of growth and mortality. Western Journal of Applied Forestry 2: 111-114.
Desai, A.R., Paw, U.K.T., Cook, B.D., Davis, K.J. and Carey, E.V. 2005. Comparing net ecosystem exchange of carbon dioxide between an old-growth and mature forest in the upper Midwest, USA. Agricultural and Forest Meteorology 128: 33-55.
Ellsworth, D.S. 2000. Seasonal CO2 assimilation and stomatal limitations in a Pinus taeda canopy with varying climate. Tree Physiology 20: 435-444.
Falk, M., Paw, U.K.T., Schroeder, M. 2002. Interannual variability of carbon and energy fluxes for an old-growth rainforest. In: Proceedings of the 25th Conference on Agricultural and Forest Meteorology. American Meteorological Society, Boston, Massachusetts, USA.
Franklin, J.F. 1988. Pacific Northwest Forests. In: Barbour, M.G. and Billings, W.D. (Eds.) North American Terrestrial Vegetation. Cambridge University Press, New York, New York, USA, pp. 104-131.
Franklin, J.F. and DeBell, D.S. 1988. Thirty-six years of tree population change in an old-growth Pseudotsuga-Tsuga forest. Canadian Journal of Forest Research 18: 633-639.
Friedlingstein, P., Cox, P., Betts, R., Bopp, L., von Bloh, W., Brovkin, V., Cadule, P., Doney, S., Eby, M., Fung, I., Bala, G., John, J., Jones, C., Joos, F., Kato, T., Kawamiya, M., Knorr, W., Lindsay, K., Matthews, H.D., Raddatz, T., Rayner, P., Reick, C., Roeckner, E., Schnitzler, K.-G., Schnur, R., Strassmann, K., Weaver, A.J., Yoshikawa, C. and Zeng, N. 2006. Climate-carbon cycle feedback analysis: Results from the (CMIP)-M-4 model intercomparison. Journal of Climate 19: 3337-3353.
Graumlich, L.J. 1991. Subalpine tree growth, climate, and increasing CO2 : an assessment of recent growth trends. Ecology 72: 1-11.
Graybill, D.A. 1987. A network of high elevation conifers in the western US for detection of tree-ring growth response to increasing atmospheric carbon dioxide. In: Jacoby, G.C. and Hornbeck, J.W., (Eds.) Proceedings of the International Symposium on Ecological Aspects of Tree-Ring Analysis. U.S. Department of Energy Conference Report DOE/CONF8608144, pp. 463-474.
Graybill, D.A. and Idso, S.B. 1993. Detecting the aerial fertilization effect of atmospheric CO2 enrichment in tree-ring chronologies. Global Biogeochemical Cycles 7: 81-95.
Greenep, H., Turnbull, M.H. and Whitehead, D. 2003. Response of photosynthesis in second-generation Pinus radiata trees to long-term exposure to elevated carbon dioxide partial pressure. Tree Physiology 23: 569-576.
Guan, D., Wu, J.B., Zhao, X.S., Han, S.J., Yu, G.R., Sun, X.M. and Jin, C.J. 2006. CO2 fluxes over an old temperate mixed forest in northeastern China. Agricultural and Forest Meteorology 137: 138-149.
Hamilton, J.G., DeLucia, E.H., George, K., Naidu, S.L., Finzi, A.C. and Schlesinger, W.H. 2002. Forest carbon balance under elevated CO2. Oecologia 10.1007/s00442-002-0884-x.
Hietz, P., Wanek, W. and Dunisch, O. 2005. Long-term trends in cellulose ð13C and water-use efficiency of tropical Cedrela and Swietenia from Brazil. Tree Physiology 25: 745-752.
Hollinger, D.Y., Kelliher, F.M., Byers, J.N., Hunt, J.E., McSeveny, T.M. and Weir, P.L. 1994. Carbon dioxide exchange between an undisturbed old-growth temperate forest and the atmosphere. Ecology 75: 143-150.
Ichii, K., Hashimoto, H., Nemani, R. and White, M. 2005. Modeling the interannual variability and trends in gross and net primary productivity of tropical forests from 1982 to 1999. Global and Planetary Change 48: 274-286.
Idso, S.B. 1995. CO2 and the Biosphere: The Incredible Legacy of the Industrial Revolution. Department of Soil, Water and Climate, University of Minnesota, St. Paul, Minnesota, USA.
Jacoby G.C. 1986. Long-term temperature trends and a positive departure from the climate-growth response since the 1950s in high elevation lodgepole pine from California. In: Rosenzweig, C. and Dickinson, R. (Eds.) Proceedings of the NASA Conference on Climate-Vegetation Interactions. Office for Interdisciplinary Earth Studies (OIES), University Corporation for Atmospheric Research (UCAR), Boulder, Colorado, USA, pp. 81-83.
Kienast, F. and Luxmoore, R.J. 1998. Tree-ring analysis and conifer growth responses to increased atmospheric CO2 levels. Oecologia 76: 487-495.
Knapp, P.A., Soule, P.T. and Grissino-Mayer, H.D. 2001. Detecting potential regional effects of increased atmospheric CO2 on growth rates of western juniper. Global Change Biology 7: 903-917.
Knohl, A., Schulze, E.-D., Kolle, O. and Buchmann, N. 2003. Large carbon uptake by an unmanaged 250-year-old deciduous forest in Central Germany. Agricultural and Forest Meteorology 118: 151-167.
Koch, G.W., Sillett, S.C., Jennings, G.M. and Davis, S.D. 2004. The limits to tree height. Nature 428: 851-854.
Lai, C.T., Katul, G., Butnor, J., Ellsworth, D. and Oren, R. 2002. Modeling nighttime ecosystem respiration by a constrained source optimization method. Global Change Biology 8: 124-141.
LaMarche Jr., V.C., Graybill, D.A., Fritts, H.C. and Rose, M.R. 1984. Increasing atmospheric carbon dioxide: tree ring evidence for growth enhancement in natural vegetation. Science 225: 1019-1021.
Laurance, S.G.W., Laurance, W.F., Nascimento, H.E.M., Andrade, A., Fearnside, P.M., Rebello, E.R.G. and Condit, R. 2009. Long-term variation in Amazon forest dynamics. Journal of Vegetation Science 20: 323-333.
Laurance, W.F., Nascimento, H.E.M., Laurance, S.G., Condit, R., D'Angelo, S. and Andrade, A. 2004. Inferred longevity of Amazonian rainforest trees based on a long-term demographic study. Forest Ecology and Management 190: 131-143.
Law, B.E., Goldstein, A.H., Anthoni, P.M., Unsworth, M.H., Panek, J.A., Bauer, M.R., Fracheboud, J.M. and Hultman, N. 2001. Carbon dioxide and water vapor exchange by young and old ponderosa pine ecosystems during a dry summer. Tree Physiology 21: 299-308.
Lewis, S.L., Lopez-Gonzalez, G., Sonke, B., Affum-Baffoe, K., Baker, T.R., Ojo, L.O., Phillips, O.L., Reitsma, J.M., White, L., Comiskey, J.A., Djuikouo K., M.-N., Ewango, C.E.N., Feldpausch, T.R., Hamilton, A.C., Gloor, M., Hart, T., Hladik, A., Lloyd, J., Lovett, J.C., Makana, J.-R., Malhi, Y., Mbago, F.M., Ndangalasi, H.J., Peacock, J., Peh, K. S.-H., Sheil, D., Sunderland, T., Swaine, M.D., Taplin, J., Taylor, D., Thomas, S.C., Votere, R. and Woll, H. 2009. Increasing carbon storage in intact African tropical forests. Nature 457: 1003-1006.
Lewis, S.L., Phillips, O.L., Baker, T.R., Lloyd, J., Malhi, Y., Almeida, S., Higuchi, N., Laurance, W.F., Neill, D.A., Silva, J.N.M., Terborgh, J., Lezama, A.T., Vásquez Martinez, R., Brown, S., Chave, J., Kuebler, C., Núñez Vargas, P. and Vinceti, B. 2004. Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long-term plots. Philosophical Transactions of the Royal Society of London Series B - Biological Sciences 359: 421-436.
Lin, G., Marino, B.D.V., Wei, Y., Adams, J., Tubiello, F. and Berry, J.A. 1998. An experimental and modeling study of responses in ecosystems carbon exchanges to increasing CO2 concentrations using a tropical rainforest mesocosm. Australian Journal of Plant Physiology 25: 547-556.
Lloyd, J. and Farquhar, G.D. 1996. The CO2 dependence of photosynthesis, plant growth responses to elevated atmospheric CO2 concentrations and their interaction with soil nutrient status. 1. General principles and forest ecosystems. Functional Ecology 10: 4-32.
Lloyd, J. and Farquhar, G.D. 2008. Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philosophical Transactions of the Royal Society B 363: 1811-1817.
Luo, Y., Medlyn, B., Hui, D., Ellsworth, D., Reynolds, J. and Katul, G. 2001. Gross primary productivity in the Duke Forest: Modeling synthesis of the free-air CO2 enrichment experiment and eddy-covariance measurements. Ecological Applications 11: 239-252.
Luo, Y., White, L.W., Canadell, J.G., DeLucia, E.H., Ellsworth, D.S., Finzi, A., Lichter, J. and Schlesinger, W.H. 2003. Sustainability of terrestrial carbon sequestration: A case study in Duke Forest with inversion approach. Global Biogeochemical Cycles 17: 10.1029/2002GB001923.
Luyssaert, S., Schulze, E.-D., Borner, A., Knohl, A., Hessenmoller, D., Law, B.E., Ciais, P. and Grace, J. 2008. Old-growth forests as global carbon sinks. Nature 455: 213-215.
Mencuccini, M., Martinez-Vilalta, J., Vanderklein, D., Hamid, H.A., Korakaki, E. and Lee, S. 2005. Size-mediated ageing reduces vigor in trees. Ecology Letters 8: 1183-1190.
Myers, D.A., Thomas, R.B. and DeLucia, E.H. 1999. Photosynthetic capacity of loblolly pine (Pinus taeda L.) trees during the first year of carbon dioxide enrichment in a forest ecosystem. Plant, Cell and Environment 22: 473-481.
Nemani, R.R., Keeling, C.D., Hashimoto, H., Jolly, W.M., Piper, S.C., Tucker, C.J., Myneni, R.B. and Running. S.W. 2003. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300: 1560-1563.
Odum, E.P. 1963. Ecology. Holt, Rinehart and Winston, New York, New York, USA.
Odum E.P. 1965. Fundamentals of Ecology. Saunders, Philadelphia, Pennsylvania, USA.
Paw U, K.T., Falk, M., Suchanek, T.H., Ustin, S.L., Chen, J., Park, Y.-S., Winner, W.E., Thomas, S.C., Hsiao, T.C., Shaw, R.H., King, T.S., Pyles, R.D., Schroeder, M. and Matista, A.A. 2004. Carbon dioxide exchange between an old-growth forest and the atmosphere. Ecosystems 7: 513-524.
Pearce, F. 1999. That sinking feeling. New Scientist 164 (2209): 20-21.
Phillips, O.L., Aragao, L.E.O.C., Lewis, S.L., Fisher, J.B., Lloyd, J., Lopez-Gonzalez, G., Malhi, Y., Monteagudo, A., Peacock, J., Quesada, C.A., van der Heijden G., Almeida, S., Amaral, I., Arroyo, L., Aymard, G., Baker, T.R., Banki, O., Blanc, L., Bonal, D., Brando, P., Chave, J., de Oliveira, A.C.A., Cardozo, N.D., Czimczik, C.I., Feldpausch, T.R., Freitas, M.A., Gloor, E., Higuchi, N., Jimenez, E., Lloyd, G., Meir, P., Mendoza, C., Morel, A., Neill, D.A., Nepstad, D., Patino, S., Penuela, M.C., Prieto, A., Ramirez, F., Schwarz, M., Silva, J., Silveira, M., Thomas, A.S., ter Steege, H., Stropp, J., Vasquez, R., Zelazowski, P., Davila, E.A., Andelman, S., Andrade, A., Chao, K.-J., Erwin, T., Di Fiore, A., Honorio C., E., Keeling, H., Killeen, T.J., Laurance, W.F., Cruz, A.P., Pitman, N.C.A., Vargas, P.N., Ramirez-Angulo, H., Rudas, A., Salamao, R., Silva, N., Terborgh, J. and Torres-Lezama, A. 2009. Drought sensitivity of the Amazon rainforest. Science 323: 1344-1347.
Phillips, N.G., Buckley, T.N. and Tissue, D.T. 2008. Capacity of old trees to respond to environmental change. Journal of Integrative Plant Biology 50: 1355-1364.
Phillips, O.L., Malhi, Y., Higuchi, N., Laurance, W.F., Nunez, P.V., Vasquez, R.M., Laurance, S.G., Ferreira, L.V., Stern, M., Brown, S. and Grace, J. 1998. Changes in the carbon balance of tropical forests: Evidence from long-term plots. Science 282: 439-442.
Roser, C., Montagnani, L., Schulze, E.D., Mollicone, D., Kolle, O., Meroni, M., Papale, D., Marchesini, L.B., Federici, S. and Valetini, R. 2002. Net CO2 exchange rates in three different successional stages of the "Dark Taiga" of central Siberia. Tellus 54: 642-654.
Schlesinger, W.H. and Lichter, J. 2001. Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2. Nature 411: 466-469.
Schulze, E.-D., Lloyd, J., Kelliher, F.M., Wirth, C., Rebmann, C., Luhker, B., Mund, M., Knohl, A., Milyuokova, I.M. and Schulze, W. 1999. Productivity of forests in the Eurosiberian boreal region and their potential to act as a carbon sink: a synthesis. Global Change Biology 5: 703-722.
Soule, P.T. and Knapp, P.A. 2006. Radial growth rate increases in naturally occurring ponderosa pine trees: a late-20th century CO2 fertilization effect? New Phytologist 171: 379-390.
Stephens, B.B., Gurney, K.R., Tans, P.P., Sweeney, C., Peters, W., Bruhwiler, L., Ciais, P., Ramonet, M., Bousquet, P., Nakazawa, T., Aoki, S., Machida, T., Inoue, G., Vinnichenko, N., Lloyd, J., Jordan, A., Heimann, M., Shibistova, O., Langenfelds, R.L., Steele, L.P., Francey, R.J. and Denning, A.S. 2007. Weak northern and strong tropical land carbon uptake from vertical profiles of atmospheric CO2. Science 316: 1732-1735.
Tan, Z.-H., Zhang, Y.-P., Schaefer, D., Yu, G.-R., Liang, N. and Song, Q.-H. 2011. An old-growth subtropical Asian evergreen forest as a large carbon sink. Atmospheric Environment 45: 1548-1554.
Woodruff, D.R., Bond, J.B. and Meinzer, F.C. 2004. Does turgor limit growth in tall trees? Plant, Cell and Environment 27: 229-236.
Last updated 24 September 2014



