Climate-alarmists claim that rising atmospheric CO2 concentrations tend to acidify seawater (reduce its pH), which in turn leads to catastrophic decreases in the calcification rates of numerous marine organisms. But are they correct? We here review the findings of recent studies pertaining to the first half of this scenario: the phenomenon of ocean acidification.
Based on four theoretical constructs -- a geochemical model, an ocean general-circulation model, an IPCC CO2 emissions scenario for the 21st century, and a projected logistic function for the burning of earth's post-21st century fossil-fuel reserves -- Caldeira and Wickett (2003) calculated that earth's atmospheric CO2 concentration could approach 2000 ppm around the year 2300, leading to a concomitant surface seawater pH reduction of 0.7 unit, a change they describe as being much more rapid and considerably greater "than any experienced in the past 300 million years," which incredibly long time interval makes the phenomenon sound truly catastrophically detrimental, especially since it is claimed by many that this "ocean acidification" phenomenon will negatively impact the process of calcification in corals and other marine life. But is the acidification phenomenon really that severe and that unprecedented?
In a special issue of Oceanography published in December of 2009, Feely et al. review what we supposedly know about the current pH status of the world's oceans, as well as what they say we can likely expect by the end of the current century. Getting right to the crux of the matter, the three researchers write in their abstract that "estimates based on the Intergovernmental Panel on Climate Change business-as-usual emission scenarios suggest that atmospheric CO2 levels could approach 800 ppm near the end of the century," and that "corresponding biogeochemical models for the ocean indicate that surface water pH will drop from a pre-industrial value of about 8.2 to about 7.8 in the IPCC A2 scenario by the end of this century." And, of course, they warn that, as a result, "the skeletal growth rates of calcium-secreting organisms will be reduced," ending with the obligatory statement that "if anthropogenic CO2 emissions are not dramatically reduced in the coming decades, there is the potential for direct and profound impacts on our living marine ecosystems."
Well that's Feely et al.'s story; but in the same issue of Oceanography, in the article that appears just before their paper, NOAA's Pieter Tans presents a much different take on the subject. His analysis begins by indicating that the anthropogenic-induced component of the air's CO2 concentration "depends primarily on the total amount emitted, not on the rate of emissions," and that "unfortunately, the IPCC reports have not helped public understanding of this fact by choosing, somewhat arbitrarily, a rather short time horizon (100 years is most commonly used) for climate forcing by CO2." Thus, "instead of adopting the common economic point of view, which, through its emphasis on perpetual growth, implicitly assumes infinite earth resources," Tans notes that the cumulative extraction of fossil-fuel carbon currently stands at about 345 gigatons of carbon (GtC ), and that there appears to be another 640 or so GtC of proven reserves, yielding a total original reserve of about 1,000 GtC, from which he proceeds with his analysis.
The figure below shows much of the past and projected history of fossil-fuel carbon utilization, together with historical and projected atmospheric CO2 concentrations out to the year 2500, as calculated by Tans. As can be seen there, his analysis indicates that the air's CO2 concentration peaks well before 2100 and at only 500 ppm, as compared to the 800 ppm that Feely et al. take from the IPCC. In addition, by the time the year 2500 rolls around, Tans' analysis indicates that the air's CO2 concentration actually drops back to about what it is today.
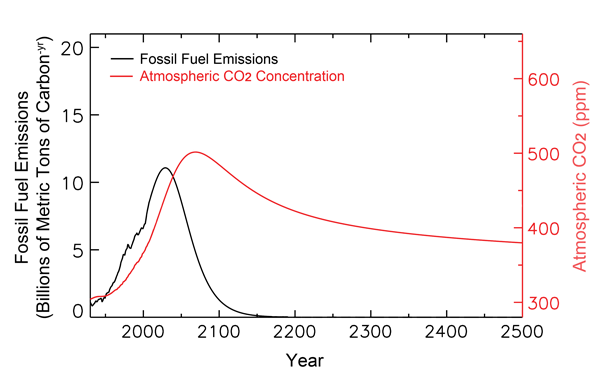
Figure 1. Past and projected trends of fossil-fuel carbon utilization and the atmosphere's CO2 concentration. Adapted from Tans (2009).
Based on his more modest projections of future atmospheric CO2 concentrations, Tans also finds the projected pH reduction of ocean waters in the year 2100 (as compared to preindustrial times) to be only one-half of the 0.4 value calculated by Feely et al., with a recovery to a reduction of only a tad over 0.1 pH unit by 2500, which is less than the range of pH values that are typical of today's oceans (8.231 in the Arctic Ocean minus 8.068 in the North Indian Ocean equals 0.163, according to Feely et al.). Clearly, therefore, things are not the way the world's climate alarmists make them out to be, especially when it comes to assessing the potential effects of anthropogenic CO2 emissions and their impacts on the air's CO2 content and oceanic pH values, an opinion that is also shared by Loaiciga (2006), who even earlier concluded that "on a global scale and over the time scales considered (hundreds of years), there would not be accentuated changes in either seawater salinity or acidity from the rising concentration of atmospheric CO2."
Another factor that can reduce the strength of the CO2-induced ocean acidification phenomenon is marine photosynthesis, which tends to increase surface seawater pH, countering the tendency for pH to decline as the air's CO2 content rises, as demonstrated by Lindholm and Nummelin (1999). This phenomenon has been found to have the ability to dramatically increase the pH of marine bays, lagoons and tidal pools (Gnaiger et al., 1978; Santhanam, 1994; Macedo et al., 2001; Hansen, 2002), as well as to significantly enhance the surface water pH of areas as large as the North Sea (Brussaard et al., 1996). And to this sizable body of research can be added the findings of Middelboe and Hansen (2007), who studied a wave-exposed boulder reef in Aalsgaarde on the northern coast of Zealand, Denmark, plus a sheltered shallow-water area in Kildebakkerne in the Roskilde Fjord, Denmark.
In this study, the two researchers report -- in line with what one would expect if photosynthesis tends to increase surface-water pH -- that (1) "daytime pH was significantly higher in spring, summer and autumn than in winter at both study sites," often reaching values of 9 or more during peak summer growth periods vs. 8 or less in winter, that (2) "diurnal measurements at the most exposed site showed significantly higher pH during the day than during the night," reaching values that sometimes exceeded 9 during daylight hours but that typically dipped below 8 at night, and that (3) "diurnal variations were largest in the shallow water and decreased with increasing water depth."
In addition to their own findings, Middelboe and Hansen cite those of (1) Pearson et al. (1998), who found that pH averaged about 9 during the summer in populations of Fucus vesiculosus in the Baltic Sea, (2) Menendez et al. (2001), who found that maximum pH was 9 to 9.5 in dense floating macroalgae in a brackish coastal lagoon in the Ebro River Delta, and (3) Bjork et al. (2004), who found pH values as high as 9.8 to 10.1 in isolated rock pools in Sweden. Noting that "pH in the sea is usually considered to be stable at around 8 to 8.2," the two Danish researchers thus concluded that "pH is higher in natural shallow-water habitats than previously thought."
In another important study, Liu et al. (2009) wrote that "the history of ocean pH variation during the current interglacial (Holocene) remains largely unknown," and that it "would provide critical insights on the possible impact of acidification on marine ecosystems." Hence, they set about to provide just such a context.
Working with eighteen samples of fossil and modern Porites corals recovered from the South China Sea, the nine researchers employed 14C dating using the liquid scintillation counting method, along with positive thermal ionization mass spectrometry to generate high precision δ11B (boron) data, from which they reconstructed the paleo-pH record of the past 7000 years that is depicted in the figure below.
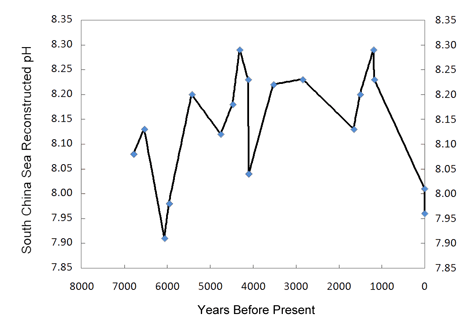
Figure 2. Reconstructed pH history of the South China Sea. Created from Table 1 of Liu et al. (2009).
As can be seen from this figure, there is nothing unusual, unnatural or unprecedented about the two most recent pH values. They are neither the lowest of the record, nor is the decline rate that led to them the greatest of the record. Hence, there is no compelling reason to believe they were significantly influenced by the nearly 40% increase in the air's CO2 concentration that occurred over the course of the Industrial Revolution. As for the prior portion of the record, Liu et al. note that there is also "no correlation between the atmospheric CO2 concentration record from Antarctica ice cores and δ11B-reconstructed paleo-pH over the mid-late Holocene up to the Industrial Revolution."
Further enlightenment comes from the earlier work of Pelejero et al. (2005), who developed a more refined history of seawater pH spanning the period 1708-1988 (depicted in the figure below), based on δ11B data obtained from a massive Porites coral from Flinders Reef in the western Coral Sea of the southwestern Pacific. These researchers also found that "there is no notable trend toward lower δ11B values." Instead, they discovered that "the dominant feature of the coral δ11B record is a clear interdecadal oscillation of pH, with δ11B values ranging between 23 and 25 per mil (7.9 and 8.2 pH units)," which they say "is synchronous with the Interdecadal Pacific Oscillation."
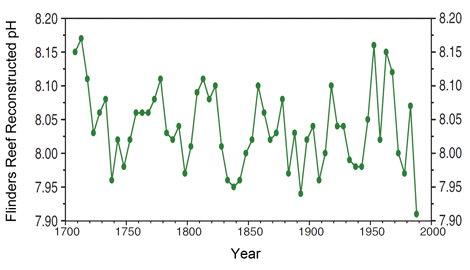
Figure 3. Reconstructed pH history of Flinders Reef of the Western Coral Sea of the Southwestern Pacific. Adapted from Pelejero et al. (2005).
Going one step further, Pelejero et al. also compared their results with coral extension and calcification rates obtained by Lough and Barnes (1997) over the same 1708-1988 time period; and as best we can determine from their graphical representations of these two coral growth parameters, extension rates over the last 50 years of this period were about 12% greater than they were over the first 50 years, while calcification rates were approximately 13% greater over the last 50 years.
More recently, Wei et al. (2009) derived the pH history of Arlington Reef (off the north-east coast of Australia) that is depicted in the figure below. As can be seen there, there was a ten-year pH minimum centered at about 1935 (which obviously was not CO2-induced) and a shorter more variable minimum at the end of the record (which also was not CO2-induced); and apart from these two non-CO2-related exceptions, the majority of the data once again fall within a band that exhibits no long-term trend, such as would be expected to have occurred if the gradual increase in atmospheric CO2 concentration since the inception of the Industrial Revolution were truly making the global ocean less basic.
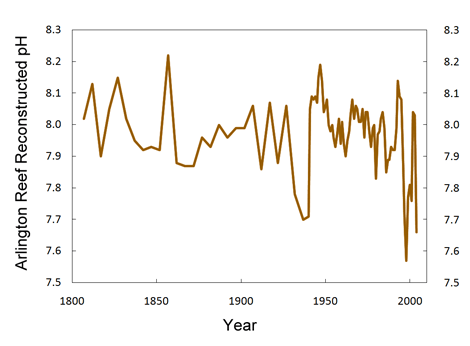
Figure 4. Reconstructed pH history of Arlington Reef off the northeast coast of Australia. Adapted from Wei et al. (2009).
In addition to all of the above observations, numerous scientific studies have demonstrated that atmospheric CO2 enrichment stimulates pH-boosting photosynthesis in both marine micro- and macro-algae. See Aquatic Plants (Marine -- Microalgae) and Aquatic Plants (Marine -Macroalgae) in our Subject Index. And in light of this phenomenon, it is logical to presume that anything else that enhances marine photosynthesis -- such as nutrient delivery to the waters of the world's coastal zones (i.e., eutrophication) -- may do so as well; and Borges and Gypens (2010) have in fact found such to be the case.
Employing an idealized biogeochemical model of a river system (Billen et al., 2001) and a complex biogeochemical model describing carbon and nutrient cycles in the marine domain (Gypens et al., 2004), the two researchers investigated "the decadal changes of seawater carbonate chemistry variables related to the increase of atmospheric CO2 and of nutrient delivery in the highly eutrophied Belgian coastal zone over the period 1951-1998." This work revealed, as they describe it, that "the increase of primary production due to eutrophication could counter the effects of ocean acidification on surface water carbonate chemistry in coastal environments," and that "changes in river nutrient delivery due to management regulation policies can lead to stronger changes in carbonate chemistry than ocean acidification," as well as changes that are "faster than those related solely to ocean acidification." And to make these facts perfectly clear, they add that "the response of carbonate chemistry to changes of nutrient delivery to the coastal zone is stronger than ocean acidification."
Once again, therefore, we have another situation where the doom-and-gloom prognostications of the world's climate alarmists have been made without regard to the full spectrum of important phenomena that come to bear upon the issue in question, and where the conclusions they reach are found to be far more uncertain and much less extreme than what they portray them to be. Thus, it can be appreciated that the climate-alarmist horror stories of impending extinctions of earth's marine calcifying organisms due to a CO2-induced decrease in seawater pH are merely that: stories, without any basis in fact.
References
Billen, G., Garnier, J., Ficht, A. and Cun, C. 2001. Modeling the response of water quality in the Seine river estuary to human activity in its watershed over the last 50 years. Estuaries 24: 977-993.
Bjork, M., Axelsson, L. and Beer, S. 2004. Why is Ulva intestinalis the only macroalga inhabiting isolated rockpools along the Swedish Atlantic coast? Marine Ecology Progress Series 284: 109-116.
Borges, A.V. and Gypens, N. 2010. Carbonate chemistry in the coastal zone responds more strongly to eutrophication than to ocean acidification. Limnology and Oceanography 55: 346-353.
Brussaard, C.P.D., Gast, G.J., van Duyl, F.C. and Riegman, R. 1996. Impact of phytoplankton bloom magnitude on a pelagic microbial food web. Marine Ecology Progress Series 144: 211-221.
Caldeira, K. and Wickett, M.E. 2003. Anthropogenic carbon and ocean pH. Nature 425: 365.
Feely, R.A., Doney, S.C. and Cooley, S.R. 2009. Ocean acidification: Present conditions and future changes in a high-CO2 world. Oceanography 22: 36-47.
Gnaiger, E., Gluth, G. and Weiser, W. 1978. pH fluctuations in an intertidal beach in Bermuda. Limnology and Oceanography 23: 851-857.
Gypens, N., Lancelot, C. and Borges, A.V. 2004. Carbon dynamics and CO2 air-sea exchanges in the eutrophied costal waters of the Southern Bight of the North Sea: A modelling study. Biogeosciences 1: 147-157.
Hansen, P.J. 2002. The effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquatic Microbiology and Ecology 28: 279-288.
Lindholm, T. and Nummelin, C. 1999. Red tide of the dinoflagellate Heterocapsa triquetra (Dinophyta) in a ferry-mixed coastal inlet. Hydrobiologia 393: 245-251.
Liu, Y., Liu, W., Peng, Z., Xiao, Y., Wei, G., Sun, W., He, J. Liu, G. and Chou, C.-L. 2009. Instability of seawater pH in the South China Sea during the mid-late Holocene: Evidence from boron isotopic composition of corals. Geochimica et Cosmochimica Acta 73: 1264-1272.
Loaiciga, H.A. 2006. Modern-age buildup of CO2 and its effects on seawater acidity and salinity. Geophysical Research Letters 33: 10.1029/2006GL026305.
Lough, J.M. and Barnes, D.J. 1997. Several centuries of variation in skeletal extension, density and calcification in massive Porites colonies from the Great Barrier Reef: A proxy for seawater temperature and a background of variability against which to identify unnatural change. Journal of Experimental and Marine Biology and Ecology 211: 29-67.
Macedo, M.F., Duarte, P., Mendes, P. and Ferreira, G. 2001. Annual variation of environmental variables, phytoplankton species composition and photosynthetic parameters in a coastal lagoon. Journal of Plankton Research 23: 719-732.
Menendez, M., Martinez, M. and Comin, F.A. 2001. A comparative study of the effect of pH and inorganic carbon resources on the photosynthesis of three floating macroalgae species of a Mediterranean coastal lagoon. Journal of Experimental Marine Biology and Ecology 256: 123-136.
Middelboe, A.L. and Hansen, P.J. 2007. High pH in shallow-water macroalgal habitats. Marine Ecology Progress Series 338: 107-117.
Pearson, G.A., Serrao, E.A. and Brawley, S.H. 1998. Control of gamete release in fucoid algae: sensing hydrodynamic conditions via carbon acquisition. Ecology 79: 1725-1739.
Pelejero, C., Calvo, E., McCulloch, M.T., Marshall, J.F., Gagan, M.K., Lough, J.M. and Opdyke, B.N. 2005. Preindustrial to modern interdecadal variability in coral reef pH. Science 309: 2204-2207.
Santhanam, R., Srinivasan, A., Ramadhas, V. and Devaraj, M. 1994. Impact of Trichodesmium bloom on the plankton and productivity in the Tuticorin bay, southeast coast of India. Indian Journal of Marine Science 23: 27-30.
Tans, P. 2009. An accounting of the observed increase in oceanic and atmospheric CO2 and an outlook for the future. Oceanography 22: 26-35.
Wei, G., McCulloch, M.T., Mortimer, G., Deng, W. and Xie, L. 2009. Evidence for ocean acidification in the Great Barrier Reef of Australia. Geochimica et Cosmochimica Acta 73: 2332-2346.
Last updated XX June 2010



